Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
High-energy battery electrodes load up on sulfur
A new lithium-sulfur battery electrode packs more energy with 10 times as much sulfur as existing designs
by Katherine Bourzac
June 15, 2016

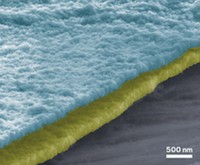
Lithium-sulfur batteries could theoretically store an order of magnitude more energy by weight than today’s lithium-ion batteries because sulfur can incorporate more lithium ions than the standard electrode materials. This could extend the range of electric vehicles and the battery life of portable electronics without adding weight—and sulfur is abundant and cheap. Now, a team of researchers has designed a cathode for such batteries that holds up to 10 times as much sulfur as other designs without a drop in performance (ACS Energy Lett. 2016, DOI: 10.1021/acsenergylett.6b00104). A test battery made with the cathode had eightfold higher energy capacity than a typical cellphone battery.
Putting sulfur in battery cathodes presents many engineering challenges. Because sulfur has low electrical conductivity, engineers usually compensate by adding conductive, carbon-based compounds to the cathode. But these materials reduce the amount of sulfur that can fit in the battery, lowering the energy density. “We want to increase the sulfur loading while maintaining good performance so that lithium-sulfur batteries can compete,” says Arumugam Manthiram of the University of Texas, Austin.
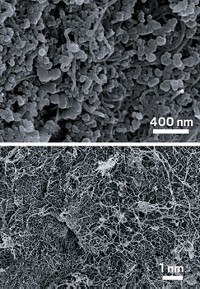
He, Yang-Kook Sun of Hanyang University, and their colleagues combined two complementary carbon materials to boost the electrode’s conductivity: acetylene black and multiwalled carbon nanotubes. The careful mix of these materials creates a balance between mechanical strength and high conductivity per volume, allowing more incorporation of elemental sulfur without decreasing the conductivity or total energy storage. Other batteries of this type have relied on sulfur compounds, lowering the total sulfur loading.
In their design, the researchers made sure to address another known issue with lithium-sulfur batteries: Sulfur reacts with lithium ions to form lithium polysulfides that can migrate out of the cathode, removing lithium from operation. Using UV-Vis spectroscopy, the researchers confirmed that when the carbon materials were doped with nitrogen, the materials bound to the polysulfides and kept them from escaping. To further help with this, the researchers used chitosan, a polysaccharide found in crustacean shells, as a binder.
To make the cathode, the researchers spread a slurry of the nitrogen-doped carbon materials, chitosan binder, and elemental sulfur on aluminum foil and topped it with a net made of nanotubes to act as a final barrier to keep polysulfides from escaping. “The production cost of our system should be low compared to others,” Sun says. “Our materials are cheap, and we are using existing manufacturing technology.”
The completed cathode contains 10 mg of sulfur per cm2—much more than the typical 1 or 2 mg/cm2, Manthiram says. When the researchers paired the cathode with a lithium-metal anode to form a battery, the device had an initial capacity of 1,332 milliamp-hours per gram. A typical cell phone battery has a capacity of about 170 mA-hr/g, Manthiram says. After discharging and charging 50 times, the new design maintains 91% of that storage capacity. After further charge cycles, the anode would start to break down; the limited lifetime of lithium-metal electrodes is another engineering challenge.
Yuegang Zhang, a battery chemist at the Chinese Academy of Sciences, says it’s significant that the researchers were able to get more sulfur in the battery. But Zhang says they need to figure out how to boost the sulfur content even further to compete with commercial technology.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter