Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Bacterial ticker tape puts cells on display
Microfluidic device shows how microbes change over generations
by Jyoti Madhusoodanan
July 14, 2016

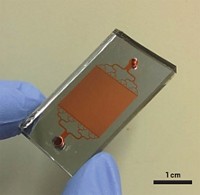
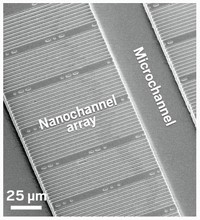
When a bacterial cell multiplies on a surface, its offspring typically stay close. Daughter cells rapidly pile up to form mounds of microbes, making it difficult to study the growth and behavior of individual cells. Researchers have now built a transparent array of nanochannels where microbes grow single file, so they can study a bacterium and its daughter cells over multiple generations (Anal. Chem. 2016, DOI: 10.1021/acs.analchem.6b00889).

Tracking single cells in this way could help identify how microbes acquire traits such as antibiotic resistance over time. “We took a three-dimensional problem and turned it into a single-dimensional one,” says study author Yves V. Brun, a biologist at Indiana University.
Brun, chemist Stephen C. Jacobson, and colleagues fabricated a ladder-shaped pattern in a polymer film on a glass slide and covered it with another glass layer. Microchannels formed the ladder’s side rails, and nanochannels several hundred nanometers wide—chosen to match the width of the bacteria they were growing—formed the rungs. Nutrient medium diffused from the microchannels into the nanochannels, so the bacteria within remained undisturbed by the fluid flowing past them.
The team seeded the nanochannels with Bacillus subtilis bacteria engineered to glow red and then used a microscope to watch them divide, confirming that the cells grew at the same rate as in standard culture. Next they studied another B. subtilis carrying a green fluorescence gene, which was controlled by a genetic element known be activated in only a fraction of cells in a population. Over five generations of growth, the team tracked which cells glowed green and which didn’t—and found that daughter cells were more likely to glow if their parent cell glowed, but that the correlation declined with every generation away from the original parent.
“Our design really simplifies both the imaging and downstream analysis,” says Jacobson. “Pretty much any question you can address by looking at a fluorescent readout is greatly facilitated by these devices.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter