Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
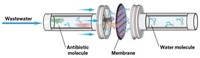
Membrane coating could help remove endocrine disruptors from wastewater
Adding a hydrophilic coating to a nanofiltration membrane helps clean parabens and bisphenol A from wastewater for recycling
by Deirdre Lockwood
September 19, 2016

Water-stressed communities from California to Singapore have begun reclaiming wastewater for drinking and other uses, but they face a challenge: It’s hard for the treatment process to remove trace organic pollutants, such as endocrine disruptors. Now researchers have developed a potential solution, coating a commonly used filtration membrane with a material that rejects endocrine-disrupting molecules (Environ. Sci. Technol. Lett. 2016, DOI: 10.1021/acs.estlett.6b00263).
Many of the commercially available nanofiltration and reverse osmosis membranes currently used to clean wastewater were optimized for desalination. They’re made with a relatively hydrophobic polyamide composite that rejects salt and other ions. Since many endocrine disruptors, such as bisphenol A (BPA) and parabens, are also hydrophobic, these compounds tend to stick to the membrane and can eventually diffuse through to the other side (Environ. Sci. Technol. 2004, DOI: 10.1021/es034952r).
Chuyang Y. Tang of the University of Hong Kong and his colleagues thought that adding a hydrophilic coating to the membranes might prevent this problem. They tested their hunch with polydopamine, a thin hydrophilic coating researchers are exploring as a way to prevent membrane fouling in wastewater treatment. The researchers coated a commercial polyamide-based nanofiltration membrane, NF90, by shaking it with a solution of dopamine hydrochloride for up to four hours; the dopamine self-polymerizes on the membrane surface. Then they tested the system’s performance in filtering water containing four endocrine disruptors: ethylparaben, propylparaben, benzylparaben, and BPA, at 200 μg/L, which is comparable to or higher than their average concentration in wastewater.
The coated membrane rejected 60 to 70% of the three paraben compounds tested, whereas the untreated membrane rejected 35 to 50%. BPA rejection improved from 96% for the uncoated membrane to 99% for the coated version.
However, the four-hour coating also lowered the membrane’s water permeability by 45%, which Tang says is too much to be practical and something his team wants to improve—a 10% reduction would be more “bearable” for application. Recently, they developed a more hydrophilic coating material that can be applied in very thin layers, minimizing the adverse effect on membrane permeability yet maintaining rejection of endocrine disruptors, according to their preliminary results. What’s more, this approach shortens the coating time from hours to minutes, “an important consideration for industrial upscaling,” Tang says.
Long Nghiem, a membrane engineer at the University of Wollongong, praises the team’s “elegant but simple approach,” while noting that it is a proof-of-concept study. He says the researchers must next assess the impact of the coating on membrane fouling.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter