Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Custom designed zeolites make better catalysts
Zeolites synthesized with transition state mimics exhibit enhanced activity and product selectivity
by Mitch Jacoby
March 14, 2017
Endowed with a network of interconnected, molecule-sized pores and channels, zeolites have been used in chemical separations, oil refining, and catalysis for decades. Yet scientists still tend to use a trial-and-error approach to find the best member of this large family of crystalline materials for a given application.
Researchers in Spain may have come up with a way to shorten and reduce the cost of that time-consuming search process. Their strategy is to tailor-make zeolite catalysts with pores that closely match the proposed transition state structure of a given reaction. They find that the customized zeolite outperforms commercial zeolite catalysts commonly used for that reaction (Science 2017, DOI: 10.1126/science.aal0121).
In one example of this approach, the team, which was led by Avelino Corma, the director of the Institute of Chemical Technology at Polytechnic University of Valencia, produced catalysts for the production of xylene from toluene. That reaction, which is carried out commercially mainly with ZSM-5 and mordenite zeolites, converts two toluene molecules to one molecule of benzene and one of the dimethylbenzene isomers (o-, m-, or p-xylene).
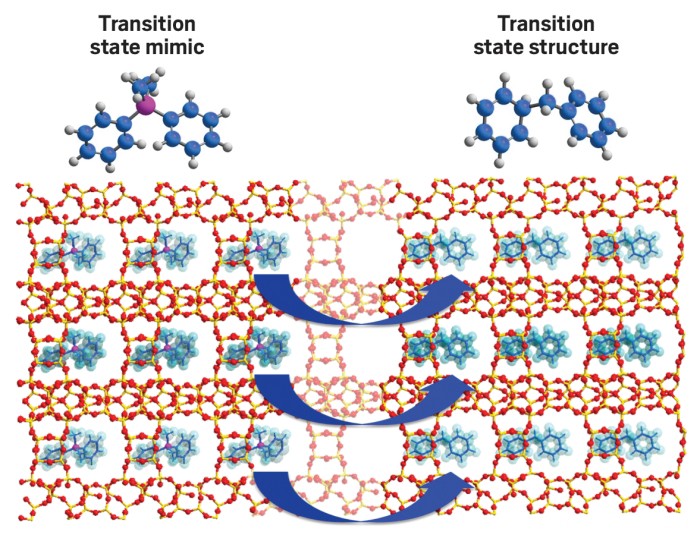
Based on earlier studies, researchers have proposed that the reaction proceeds by way of a transition state featuring a complex made of two phenyl rings joined by a positively charged carbon atom. A zeolite catalyst would need pores big enough to accommodate that structure, which is larger than the reactants and the products.
So the Valencia team selected a diphenylphosphonium compound that closely mimics the structure and charge of the transition state and used it as a structure directing agent (SDA) to synthesize a zeolite. All zeolites are aluminosilicate minerals. The team produced their zeolite catalyst, called ITQ-27, by allowing the mineral to crystallize around the diphenylphosphonium molecules. They then heated the zeolite to remove the organic material, leaving an aluminosilicate frame with pores matching the SDA and the transition state structure.
Then the team compared the performance of ITQ-27 with that of ZSM-5, mordenite, and other zeolites. They found that although some of the zeolites had a larger number of catalytic sites than ITQ-27, the custom-made ITQ-27 was the most active catalyst. It was also as selective or more selective, meaning it produced fewer by-products, than the other catalysts.
In another example of the transition state strategy, Corma and coworkers successfully synthesized zeolites for ethylbenzene isomerization.
This approach, which represents “a new paradigm in zeolite science, may accelerate discovery of highly active and selective zeolite catalysts,” says Roberto Millini, a specialist in materials chemistry and catalysis at Eni, an oil and gas company based in Italy.
Millini notes that in addition to improving the efficiency of petrochemical processes, this strategy may enable new applications for crystalline porous materials. For example, these materials may provide environmentally benign routes for converting biomass to biofuels and chemicals.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter