Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
ACS Meeting News: Biomaterials reprogram immune cells to be more tolerant
by Michael Torrice
April 5, 2017
Credit: ACS Nano
Sometimes our immune cells get overzealous, mistake parts of our own bodies for invading pathogens, and launch an attack. This is how autoimmune diseases develop. For example, in multiple sclerosis, immune cells destroy the insulating protein sheath on nerve cells, causing patients to slowly lose motor function.
At the American Chemical Society national meeting in San Francisco on Tuesday, researchers reported two biomaterials designed to tell rogue immune cells to stand down.
These two types of particles could lead to a better understanding of the mechanisms behind autoimmune diseases and possibly to novel treatments with fewer side effects, said Christopher M. Jewell of the University of Maryland, College Park, who led the work.
Current treatments for autoimmune diseases act on cells across the entire immune system, not just the malfunctioning immune cells attacking the body. This suppresses normal parts of patients’ immune systems, making people vulnerable to actual pathogens. “We’d like to have better control over the immune responses we activate and suppress,” Jewell said.
He and his colleagues are working on biomaterials that can deliver multiple molecules at once to reprogram those immune responses specific to autoimmune diseases. Previous studies have shown that particles delivering combinations of proteins targeted by the immune system along with immune-regulating molecules can induce tolerance in immune cells. Jewell presented two examples of new biomaterials during a session sponsored by the Polymeric Materials Science & Engineering Division.
Related story: Tiny technology promising for MS
In one, his team designed poly(lactic-co-glycolic acid) microparticles that can be injected directly into lymph nodes—tissues that coordinate immune cell functions. The particles are too big to drain from the nodes, so as they slowly degrade, they release their payload just within that local area, avoiding effects elsewhere in the immune system.
The particles carried two molecules: rapamycin, which regulates immune cell signaling, and a fragment of myelin, the protein that forms the insulating sheath on nerve cells. Together, these molecules reprogrammed immune cells in the lymph node to stop attacking myelin and instead told inflammatory cells targeting the protein to calm down. A single injection permanently reversed paralysis in mice with multiple sclerosis-like symptoms (Cell Reports 2016, DOI: 10.1016/j.celrep.2016.08.033).
But Jewell pointed out that polymers used to make micro- or nanoparticles sometimes can trigger immune responses or inflammation themselves. So he and his colleagues wondered whether they could develop a strategy that didn’t involve traditional biomaterials.
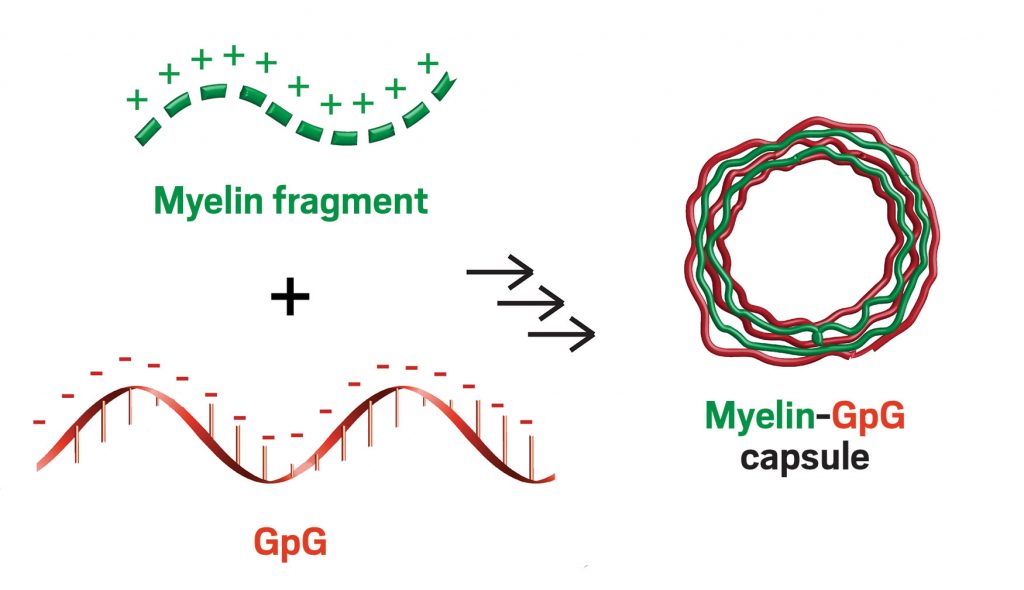
The particles they came up with consist of just a pair of immune-reprogramming molecules. The team used a layer-by-layer assembly method to build these materials, starting with a negatively charged calcium carbonate microparticle as a template. Then the researchers alternated between applying a coating of a positively charged myelin fragment and a layer of a negatively charged nucleic acid called GpG, which shuts down signaling for a toll-like receptor. This class of receptors is important in programming the functions of immune cells.
After applying a few layers, the researchers dissolved the calcium carbonate particles, leaving behind hollow capsules made from just myelin and GpG. Electrostatic interactions between the layers were strong enough to keep them together.
“The capsule is the cargo,” Jewell said. “If you have a traditional polymeric particle loaded with some small molecule drug, then maybe 1-10% is the drug. Here, 100% of the capsule is the drug.”
When injected under the skin of mice with multiple sclerosis-like symptoms, the capsules reduced inflammation caused by immune attacks on myelin and prevented the development of paralysis in all animals. The researchers observed a similar reduction in inflammatory responses when they incubated the capsules with cells taken from patients with multiple sclerosis (ACS Nano 2016, DOI: 10.1021/acsnano.6b04001).
Zhen Gu of the University of North Carolina, Chapel Hill, and North Carolina State University said both materials hold promise for clinical translation. In particular, he noted that the simple, straightforward layer-by-layer assembly of the hollow capsules makes those materials attractive for large-scale production. Such particles also could be used in immunotherapies that coax the immune system into attacking tumors, Gu said.
Another related story:


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter