Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Detergent-based artificial tongue identifies bottled water brands
An assembly containing a single type of fluorescent sensor can detect 13 different metal ions
by Prachi Patel
December 11, 2017

A new, easy-to-make artificial tongue can distinguish different brands of bottled water (ACS Sensors 2017, DOI: 10.1021/acssensors.7b00634). The simple chemical sensor uses a single type of fluorescent molecule to detect and quantify 13 different metal ions.
Fluorescent sensors are excellent for detecting minute, nanomolar levels of target chemicals in solution in real time. In these applications, molecules typically include two parts—a receptor portion that binds to the analyte connected to a fluorescent dye. When the receptor binds to its target, it triggers a change in the dye’s light emission color, intensity, or duration that can be used to quantify the target molecule’s concentration.
For these types of sensing applications, researchers have typically used arrays of different sensor molecules, each in separate wells in a dish, to measure the levels of multiple kinds of molecules in a sample. Using such sensor arrays, researchers have made artificial tongues that can identify different soft drinks, whiskeys, and wines. They could also allow authorities to monitor water quality and permit consumers to test grocery store produce for heavy-metal contamination.
But the arrays are tedious to make and require large amounts of test samples, says Liping Ding, a professor of chemistry at Shaanxi Normal University in Xi’an. So Ding and her colleagues have been looking at ways to simplify the chemical sensors.
Their new sensor uses a single sensing molecule that can fluoresce at four different wavelengths, depending on the wavelength of light applied to the sample. As different metal ions bind, they change the fluorescence pattern in measurable ways, producing an output that allows researchers to distinguish them in solution.
The fluorescent part of the sensor is a pyrene-based dye that the researchers attach to an amine-based functional group that binds metal ions. When added to water along with the common detergent, sodium dodecyl sulfate, the detergent molecules aggregate to create tiny spheres with negatively charged, water-loving surfaces and water-repelling cores. The aggregates incorporate the sensor molecules: The hydrophobic pyrene portion of the molecule becomes trapped within the spheres while the hydrophilic, metal-binding end points outward into solution.
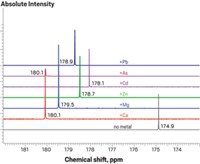
When metal ions are added to the solution, they bind to the metal receptors and interact with the anionic detergent molecules, changing the size and shape of the aggregates. This structural change in turn increases or decreases the intensity of fluorescence at each of the four wavelengths. Combining the changes at different wavelengths generates a recognition pattern for a particular metal ion, says Ding. The pattern of changes varies for each metal.
The researchers were able to record unique recognition patterns that serve as fingerprints for 13 different metal ions: Cu2+, Co2+, Ni2+, Cr3+, Hg2+, Fe3+, Zn2+, Cd2+, Al3+, Pb2+, Ca2+, Mg2+, and Ba2+. By applying a pattern recognition algorithm to the combined fluorescent signals, they could discern multiple metal ions in a mixture.
Brands of bottled mineral waters have their own signature combination and amounts of metal ions, making them an ideal test of the new sensor. When the researchers tested such samples with their sensor, they could tell tap water from mineral water and distinguish among eight different mineral water brands. The sensor could eventually be used to monitor the quality of drinking water or to detect counterfeit bottled water, Ding says.
“This is one probe for all, like ‘One ring rules them all,’” says Pavel Anzenbacher, a professor of chemistry at Bowling Green State University. “This would save a lot of work on synthesis.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter