Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Diagnosing Ebola immunity with a paper test
Antibody-detecting test strips that can be read with smartphones hold promise for disease monitoring and vaccine development
by Prachi Patel
January 24, 2018

To combat deadly outbreaks of Ebola, researchers need a variety of simple, portable tests that help them control and treat infections. Now researchers have developed a paper-based strip that detects immunity against this viral infection (ACS Nano 2018, DOI: 10.1021/acsnano.7b07021). The new test uses a color-changing paper strip similar to store-bought pregnancy kits and is read using a smartphone.

Traditional lab tests for detecting the Ebola virus require advanced facilities and take days to give results. Scientists have recently developed strip-based tests that directly detect the Ebola virus by spotting the antigens it produces. But right now, doctors don’t have access to a fast, field-based test for detecting the antibodies that humans produce to fight those antigens. “Understanding a survivor’s immune response to the disease could be really useful in understanding the spread of the disease and in helping with future management,” says Molly M. Stevens, a professor of biomedical materials & regenerative medicine at Imperial College London.
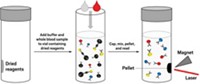
Stevens and her colleagues made their new test using commercially available paper test strips. They engineered three antigens produced by different subtypes of the Ebola virus and printed lines of the protein solutions on one end of the strips. To perform the test, a few microliters of blood serum are loaded on the other end of the strip, followed by a solution of selective antibodies that will bind to the target Ebola antibodies and label them with 40-nm-wide gold nanoparticles.
The serum and the antibody solution wick along a thin channel towards the protein test lines. Ebola antibodies in the serum sample bind to the gold nanoparticles and then to the lines of proteins. The nanoparticles aggregate and reflect light, turning the test lines reddish-purple within 15 minutes.
A smartphone app developed by the team measures the intensity of the line colors from a photograph of the test strip. An intensity above a preset threshold—determined using serum from non-infected volunteers—indicates positive for Ebola antibodies. The app also allows clinicians to add patient details and geographical location to help create a map to observe disease location and spread.
The team validated the method using serum samples from 121 people in Uganda: 90 Ebola survivors and 31 non-infected local residents. Compared with results from standard lab-based enzyme-linked immunosorbent assay (ELISA), the test was 100% accurate at detecting individuals who had survived Ebola, and gave one false positive with an uninfected sample. The test strips worked with both fresh and thawed serum samples and after being stored for 16 weeks in low humidity at room temperature. The team is now working on a whole-blood test that wouldn’t require isolating the serum to make it even easier to use in remote areas.
Medical workers in rural clinics could use the new test to identify an Ebola patient’s immune response to the disease. “Understanding who is more likely and less likely to survive could help to decide on the best treatment and care,” Stevens says. It could, for instance, help determine how best to allocate limited resources.
What’s more, the system offers a way for researchers to help control the spread of the disease by monitoring and mapping it, and even prevent future outbreaks by developing vaccines. “If an Ebola vaccine were developed, the test would also be useful for identifying individuals who would not need immunization,” says Lee Gehrke, a professor of microbiology & immunology at Massachusetts Institute of Technology, who was not involved in the work.
The researchers still need to validate the test with a larger population of survivors located across a broader area, says Kimberly Hamad-Schifferli, professor of mechanical & biological engineering at MIT, who also was not involved in the study. “Other regions may have new forms of the virus that are slightly different.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter