New Drugs Slideshow

Trulance (Peptide)

Credit: Synergy Pharmaceuticals
Active ingredient: Plecanatide
Applicant: Synergy Pharmaceuticals
Indication: Chronic idiopathic constipation
Mechanism of action Guanylate cyclase-C agonist
Wholesale acquisition cost*: $353/month
Parsabiv (Peptide)
Credit: Amgen
Active ingredient: Etelcalcetide
Applicant: Amgen
Indication: Secondary hyperparathyroidism in chronic kidney disease
Mechanism of action Calcium-sensing receptor modulator
Wholesale acquisition cost*: N/A
Emflaza (Small Molecule)
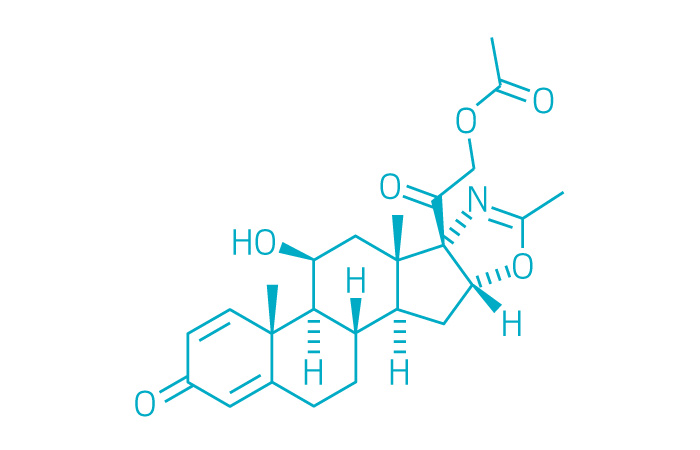
Active ingredient: Deflazacort
Applicant: Marathon Pharmaceuticals
Indication: Duchenne muscular dystrophy
Mechanism of action Corticosteroid prodrug
Wholesale acquisition cost*: $35,000/year (lowered from $89,000)
Incentive: Rare pediatric priority review voucher
Siliq (Antibody)

Credit: Valeant Pharmaceuticals
Active ingredient: Brodalumab
Applicant: Valeant Pharmaceuticals
Indication: Psoriasis
Mechanism of action IL-17RA antagonist
Wholesale acquisition cost*: $3,500/month
Xermelo (Small Molecule)
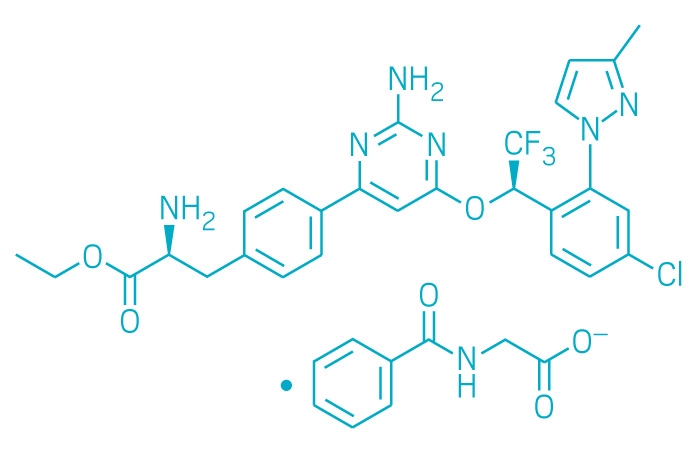
Active ingredient: Telotristat ethyl
Applicant: Lexicon Pharmaceuticals
Indication: Carcinoid syndrome diarrhea
Mechanism of action Tryptophan hydroxylase inhibitor
Wholesale acquisition cost*: $5,164/month
Kisqali (Small Molecule)
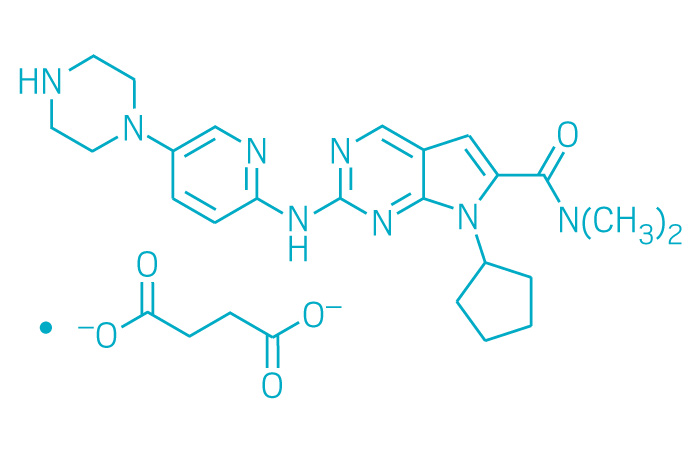
Active ingredient: Ribociclib
Applicant: Novartis
Indication: HR-positive/HER2-negative advanced or metastatic breast cancer
Mechanism of action CDK4/6 inhibitor
Wholesale acquisition cost*: 28-day supply of 600 mg pills is $10,950; 400 mg is $8,760; and 200 mg is $4,380
FDA special status: Breakthrough
Xadago (Small Molecule)
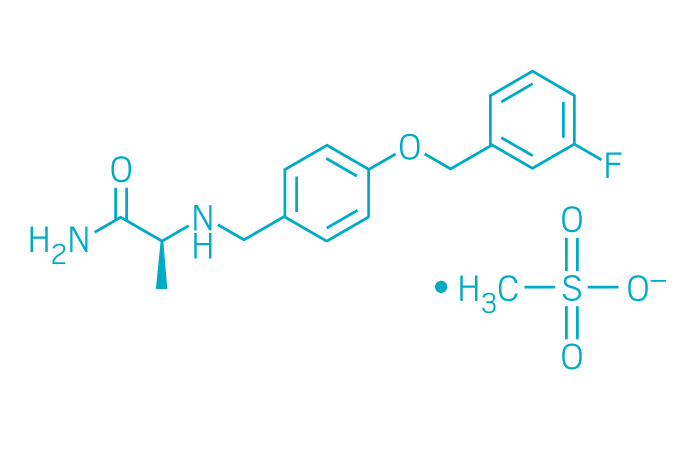
Active ingredient: Safinamide
Applicant: Newron Pharmaceuticals
Indication: Parkinson's disease
Mechanism of action Monoamine oxidase B inhibitor
Wholesale acquisition cost*: $670/month
Bavencio (Antibody)
Credit: Merck KGaA/Pfizer
Active ingredient: Avelumab
Applicant: Merck KGaA
Indication: Merkel cell carcinoma
Mechanism of action PD-L1 inhibitor
Wholesale acquisition cost*: $13,000/month
FDA special status: Breakthrough| Accelerated approval
Symproic (Small Molecule)
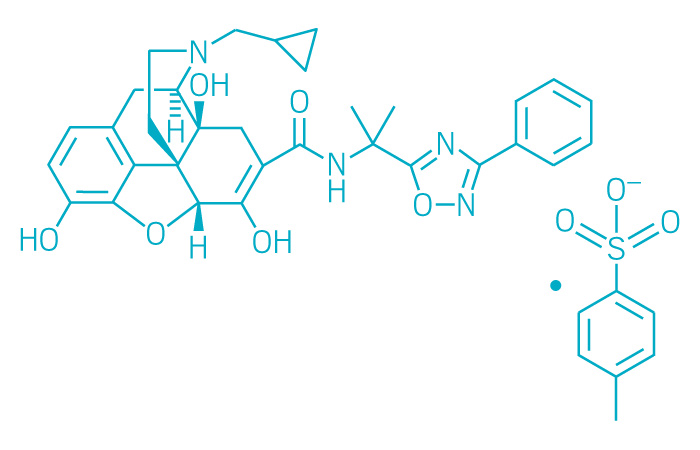
Active ingredient: Naldemedine
Applicant: Purdue Pharma
Indication: Opioid-induced constipation
Mechanism of action Mu opioid receptor antagonist
Wholesale acquisition cost*: N/A
Zejula (Small Molecule)
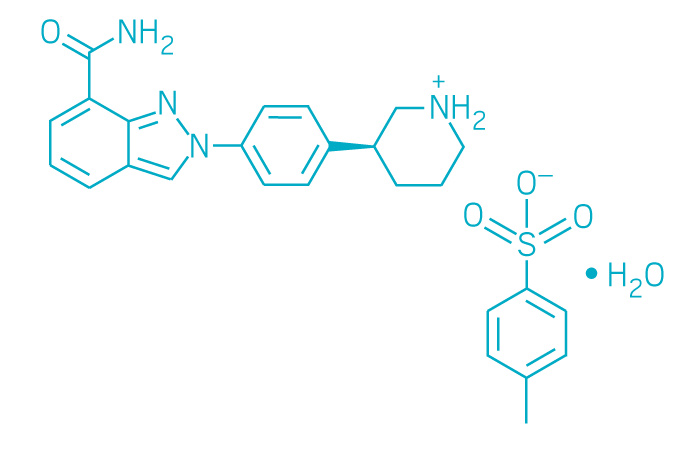
Active ingredient: Niraparib
Applicant: Tesaro
Indication: Recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer
Mechanism of action PARP inhibitor
Wholesale acquisition cost*: $9,833/month for 200 mg/day (recommended 300 mg/day dose)
FDA special status: Breakthrough
Dupixent (Antibody)
Credit: Sanofi/Regeneron Pharmaceuticals
Active ingredient: Dupilumab
Applicant: Sanofi/Regeneron Pharmaceuticals
Indication: Eczema
Mechanism of action IL-4 and IL-13 inhibitor
Wholesale acquisition cost*: $37,000/year
FDA special status: Breakthrough
Ocrevus (Antibody)
Credit: Roche
Active ingredient: Ocrelizumab
Applicant: Roche
Indication: Multiple sclerosis
Mechanism of action CD20 binder
Wholesale acquisition cost*: $65,000/year
FDA special status: Breakthrough
Austedo (Small Molecule)
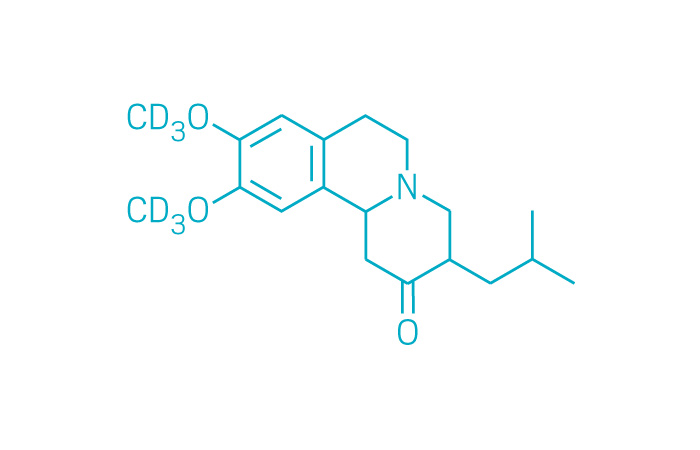
Active ingredient: Deutetrabenazine
Applicant: Teva Pharmaceutical
Indication: Huntington's disease-associated chorea
Mechanism of action Vesicular monoamine transporter 2 inhibitor
Wholesale acquisition cost*: $60,000/year
Ingrezza (Small Molecule)
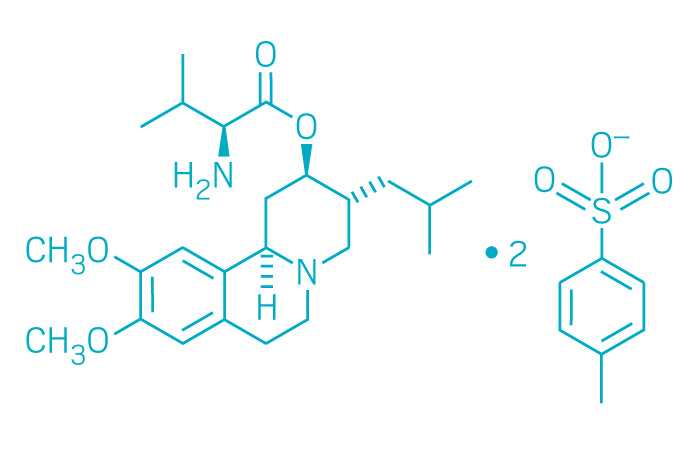
Active ingredient: Valbenazine
Applicant: Neurocrine Biosciences
Indication: Tardive dyskinesia
Mechanism of action Vesicular monoamine transporter 2 inhibitor
Wholesale acquisition cost*: $5,275/month
FDA special status: Breakthrough
Brineura (Enzyme)

Credit: BioMarin Pharmaceutical
Active ingredient: Cerliponase alfa
Applicant: Biomarin Pharmaceutical
Indication: Batten disease
Mechanism of action Enzyme replacement therapy
Wholesale acquisition cost*: $702,000/year
FDA special status: Breakthrough
Incentive: Rare pediatric priority review voucher, sold for $125 million
Alunbrig (Small Molecule)
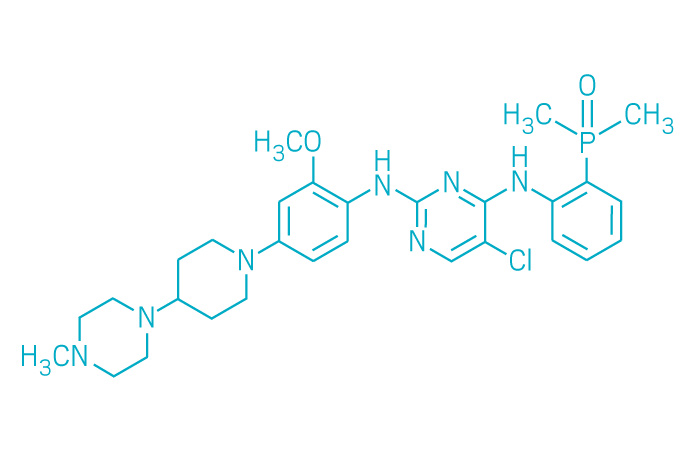
Active ingredient: Brigatinib
Applicant: Takeda Pharmaceutical
Indication: ALK-positive non-small cell lung cancer
Mechanism of action ALK inhibitor
Wholesale acquisition cost*: $14,250/month
FDA special status: Breakthrough | Accelerated approval
Rydapt (Small Molecule)
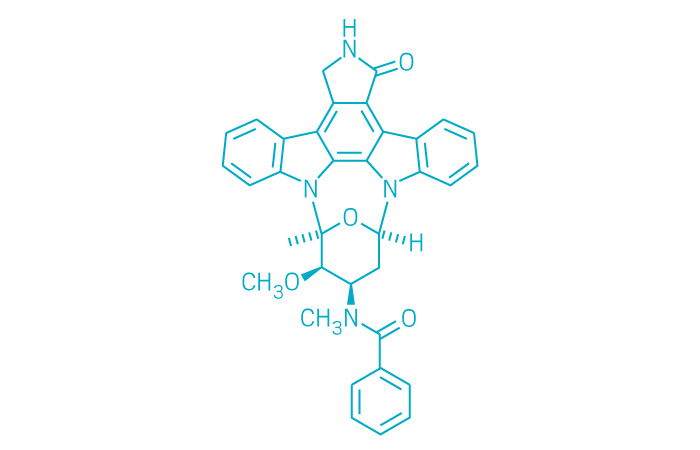
Active ingredient: Midostaurin
Applicant: Novartis
Indication: FLT3-positive acute myeloid leukemia
Mechanism of action Multikinase inhibitor, including FLT3 and KIT
Wholesale acquisition cost*: $14,990/28 days
FDA special status: Breakthrough
Tymlos (Peptide)
Credit: Radius Health
Active ingredient: Abaloparatide
Applicant: Radius Health
Indication: Osteoporosis
Mechanism of action Parathyroid hormone-related protein
Wholesale acquisition cost*: $19,500/year
Imfinzi (Antibody)

Credit: AstraZeneca
Active ingredient: Durvalumab
Applicant: AstraZeneca
Indication: Urothelial carcinoma
Mechanism of action PD-L1 inhibitor
Wholesale acquisition cost*: $180,000/year
FDA special status: Breakthrough | Accelerated approval
Radicava (Small Molecule)
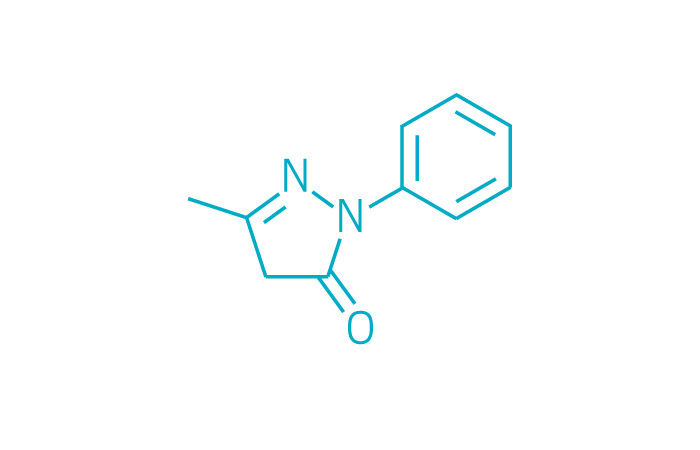
Active ingredient: Edaravone
Applicant: Mitsubishi Tanabe
Indication: Amyotrophic lateral sclerosis
Mechanism of action Unknown
Wholesale acquisition cost*: $145,524/year
Kevzara (Antibody)

Credit: Sanofi/Regeneron Pharmaceuticals
Active ingredient: Sarilumab
Applicant: Sanofi/Regeneron Pharmaceuticals
Indication: Rheumatoid arthritis
Mechanism of action IL-6R inhibitor
Wholesale acquisition cost*: $39,000/year
Baxdela (Small Molecule)
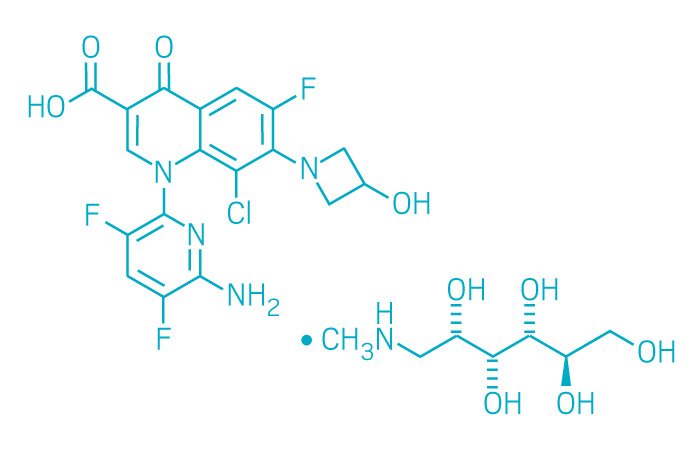
Active ingredient: Delafloxacin
Applicant: Melinta Therapeutics
Indication: Skin infections
Mechanism of action Fluoroquinolone
Wholesale acquisition cost*: N/A
Bevyxxa (Small Molecule)
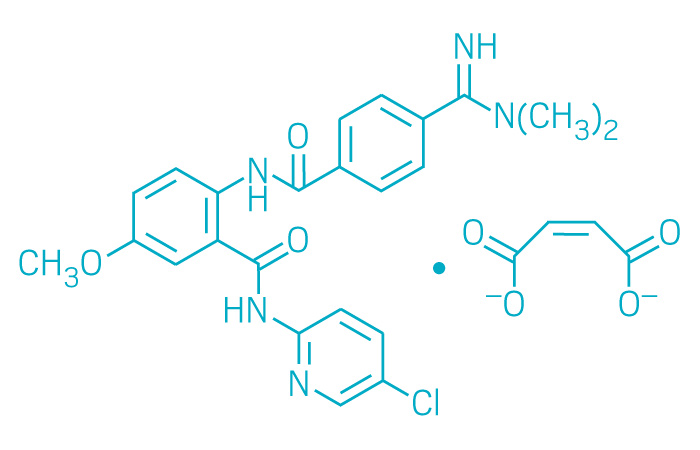
Active ingredient: Betrixaban
Applicant: Portola Pharmaceuticals
Indication: Venous thromboembolism
Mechanism of action Factor Xa inhibitor
Wholesale acquisition cost*: N/A
Tremfya (Antibody)
Credit: Johnson & Johnson
Active ingredient: Guselkumab
Applicant: Johnson & Johnson
Indication: Psoriasis
Mechanism of action IL-23 inhibitor
Wholesale acquisition cost*: $58,100/year
Incentive: Priority review voucher filed with application
Nerlynx (Small Molecule)
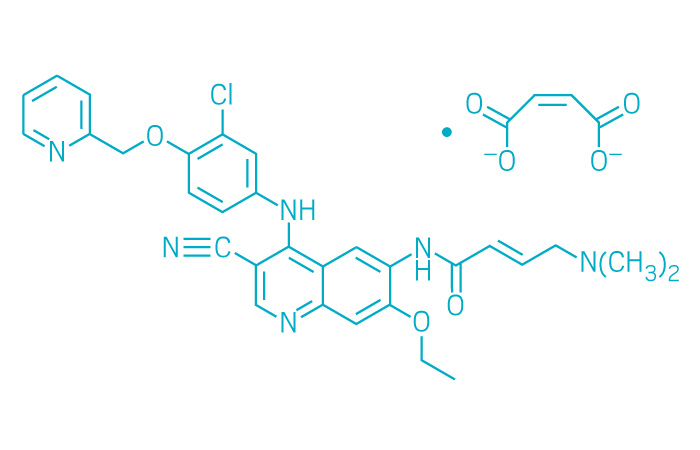
Active ingredient: Neratinib maleate
Applicant: Puma Biotechnology
Indication: HER2-positive breast cancer
Mechanism of action EGFR, HER2, and HER4 inhibitor
Wholesale acquisition cost*: $10,500/month
Vosevi (Small Molecule)
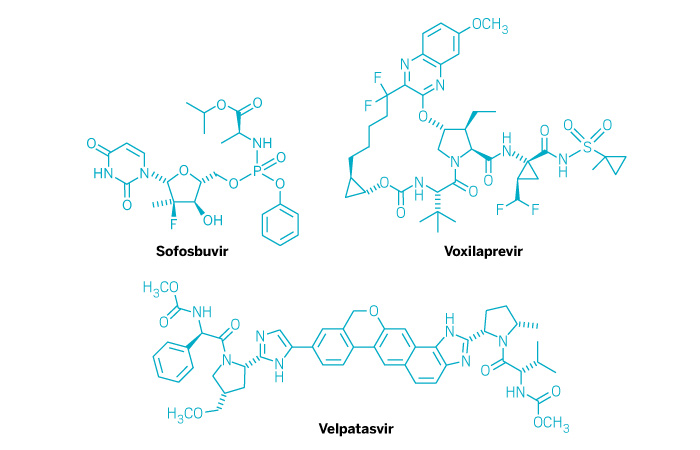
Active ingredient: Sofosbuvir, velpatasvir, and voxilaprevir
Applicant: Gilead Sciences
Indication: Hepatitis C
Mechanism of action NS5B polymerase, NS5B, and NS3/4A protease inhibitors
Wholesale acquisition cost*: $74,760/12-week course
FDA special status: Breakthrough
Idhifa (Small Molecule)
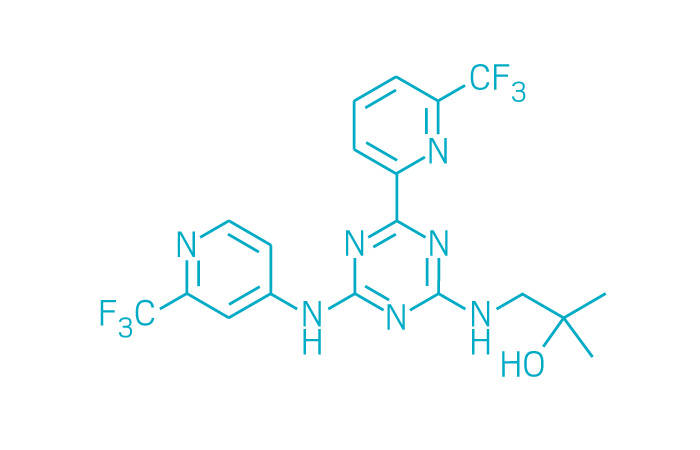
Active ingredient: Enasidenib
Applicant: Celgene
Indication: IDH2-positive acute myeloid leukemia
Mechanism of action IDH2 inhibitor
Wholesale acquisition cost*: $24,872/month
Mavyret (Small Molecule)
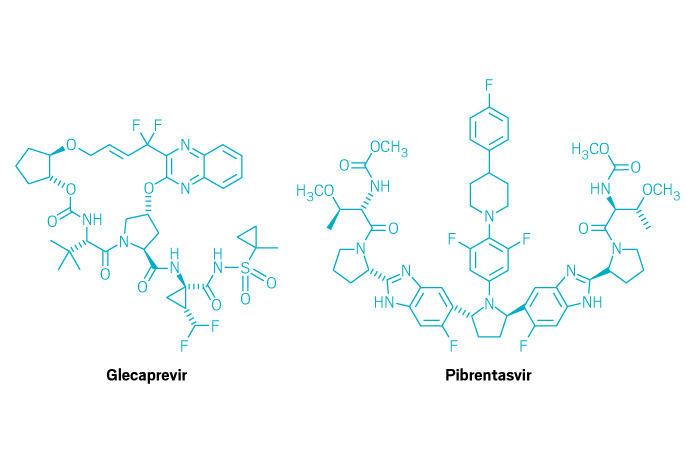
Active ingredient: Glecaprevir and pibrentasvir
Applicant: AbbVie
Indication: Hepatitis C
Mechanism of action NS3/4A protease and NS5A inhibitors
Wholesale acquisition cost*: $26,400/8-week course
FDA special status: Breakthrough
Besponsa (Antibody-Drug Conjugate)

Credit: Pfizer
Active ingredient: Inotuzumab ozogamicin
Applicant: Pfizer
Indication: Acute lymphoblastic leukemia
Mechanism of action CD22-binding antibody and cytotoxic antibiotic
Wholesale acquisition cost*: N/A
FDA special status: Breakthrough
Vabomere (Small Molecule)
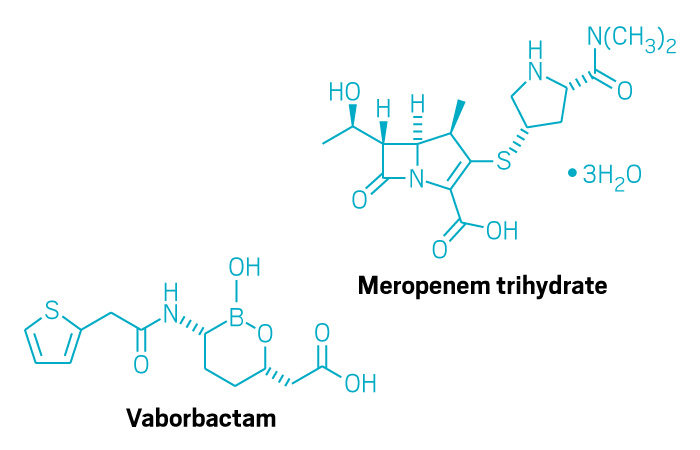
Active ingredient: Meropenem and vaborbactam
Applicant: The Medicines Co.
Indication: Complicated urinary tract infections
Mechanism of action Carbapenem antibacterial and non-β-lactam β-lactamase inhibitor
Wholesale acquisition cost*: N/A
Benznidazole (Small Molecule)
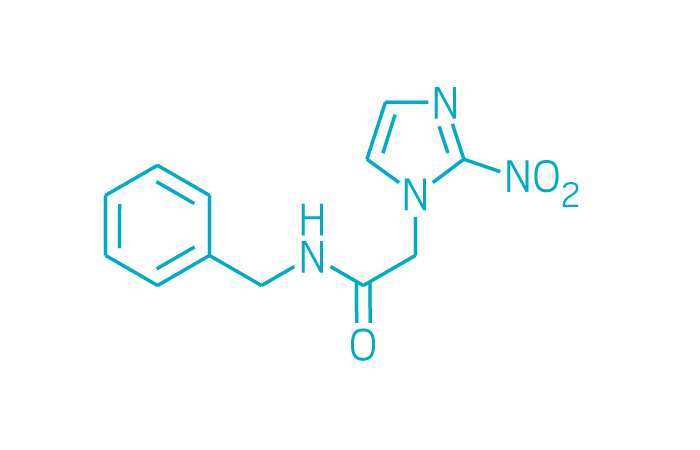
Active ingredient: Benznidazole
Applicant: Chemo Research
Indication: Chagas disease
Mechanism of action Nitroimidazole antimicrobial
Wholesale acquisition cost*: N/A
FDA special status: Accelerated approval
Incentive: Tropical disease priority review voucher
Aliqopa (Small Molecule)
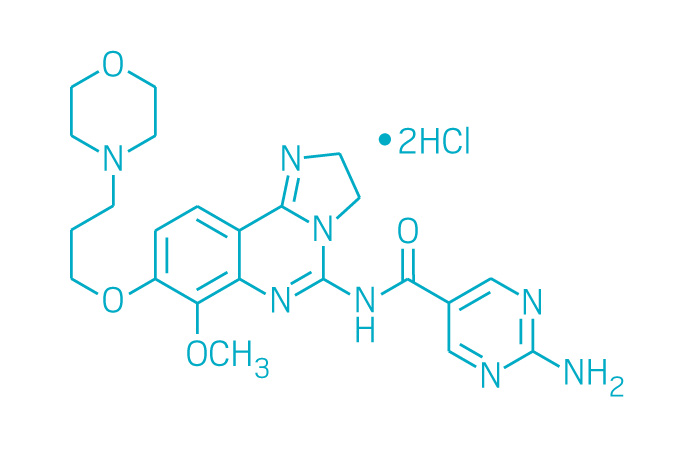
Active ingredient: Copanlisib
Applicant: Bayer
Indication: Follicular lymphoma
Mechanism of action Pan-PI3K inhibitor
Wholesale acquisition cost*: $12,726/month
FDA special status: Accelerated approval
Solosec (Small Molecule)
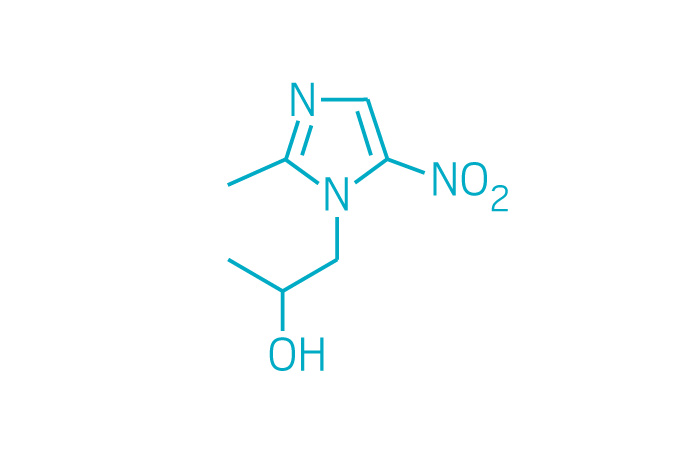
Active ingredient: Secnidazole
Applicant: Symbiomix Therapeutics
Indication: Bacterial vaginosis
Mechanism of action Nitroimidazole antimicrobial
Wholesale acquisition cost*: N/A
Verzenio (Small Molecule)
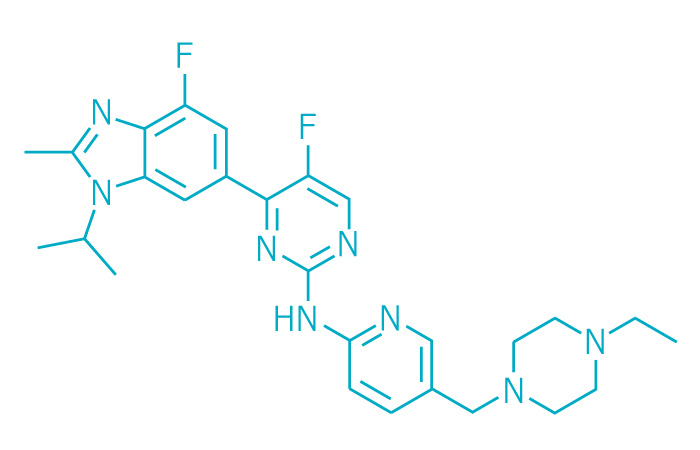
Active ingredient: Abemaciclib
Applicant: Eli Lilly & Co.
Indication: Breast cancer
Mechanism of action CDK4/6 inhibitor
Wholesale acquisition cost*: $10,948/month
FDA special status: Breakthrough
Calquence (Small Molecule)
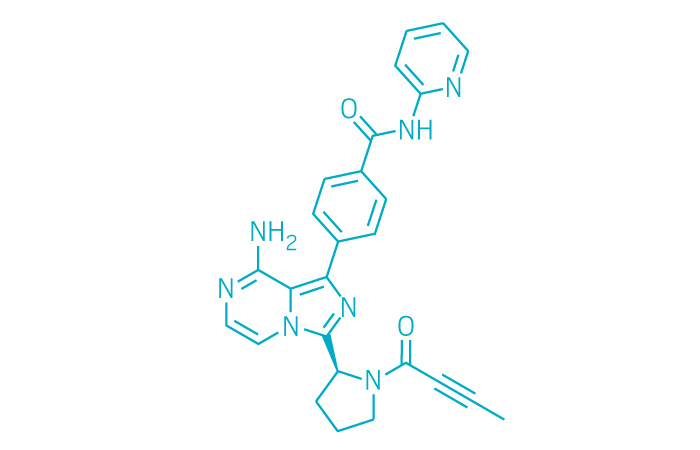
Active ingredient: Acalabrutinib
Applicant: AstraZeneca
Indication: Mantle cell lymphoma
Mechanism of action BTK inhibitor
Wholesale acquisition cost*: $14,259/month
FDA special status: Breakthrough | Accelerated approval
Vyzulta (Small Molecule)
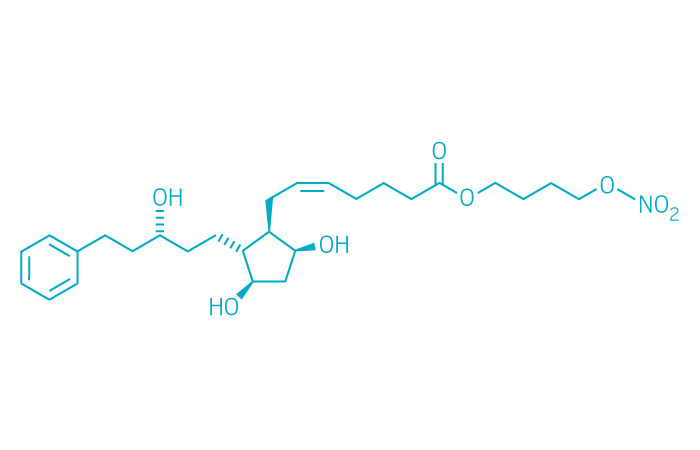
Active ingredient: Latanoprostene bunod ophthalmic solution
Applicant: Valeant Pharmaceuticals
Indication: Glaucoma/ocular hypertension
Mechanism of action Metabolizes into two pressure-lowering moieties
Wholesale acquisition cost*: N/A
Prevymis (Small Molecule)
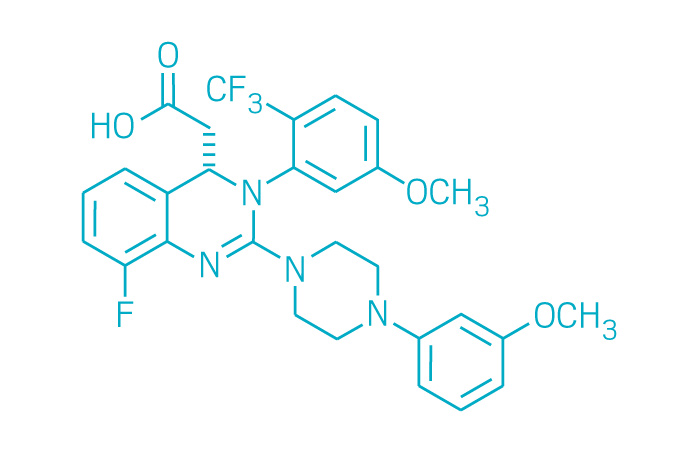
Active ingredient: Letermovir
Applicant: Merck & Co.
Indication: Infection prevention after bone marrow transplant
Mechanism of action Cytomegalovirus DNA terminase complex inhibitor
Wholesale acquisition cost*: $195/day for tablets or $270/injection
FDA special status: Breakthrough
Fasenra (Antibody)

Credit: AstraZeneca
Active ingredient: Benralizumab
Applicant: AstraZeneca
Indication: Severe asthma
Mechanism of action IL-5R α receptor binder
Wholesale acquisition cost*: $38,000/first year
Mepsevii (Enzyme)

Credit: Ultragenyx Pharmaceutical
Active ingredient: Vestronidase alfa
Applicant: Ultragenyx Pharmaceutical
Indication: MPS VII (Sly syndrome)
Mechanism of action Enzyme replacement therapy
Wholesale acquisition cost*: $375,000/year
Incentive: Rare disease priority review voucher, sold to Novartis for $130 million
Hemlibra (Bispecific Antibody)
Credit: Roche
Active ingredient: Emicizumab
Applicant: Roche
Indication: Hemophilia A
Mechanism of action Factor IX and X binder
Wholesale acquisition cost*: $482,000/first year
FDA special status: Breakthrough
Ozempic (Peptide)
Credit: Novo Nordisk
Active ingredient: Semaglutide
Applicant: Novo Nordisk
Indication: Type 2 diabetes
Mechanism of action GLP-1 receptor agonist
Wholesale acquisition cost*: $676/4–6 week supply
Xepi (Small Molecule)
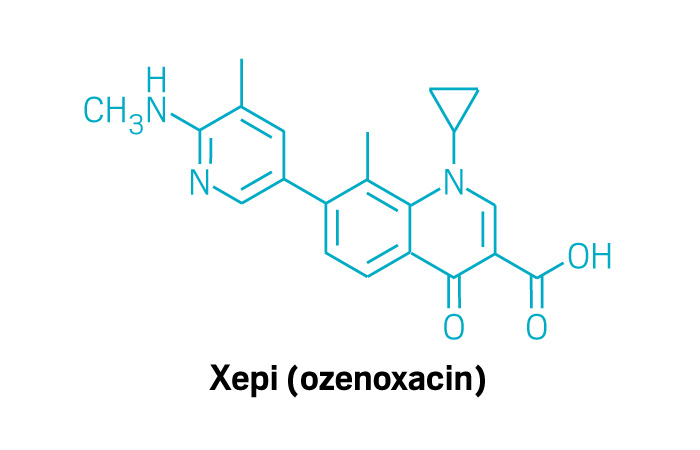
Active ingredient: Ozenoxacin
Applicant: Medimetriks Pharmaceuticals
Indication: Impetigo
Mechanism of action Nonfluorinated quinolone antibiotic
Wholesale acquisition cost*: N/A
Rhopressa (Small Molecule)
Active ingredient: Netarsudil ophthalmic solution
Applicant: Aerie Pharmaceuticals
Indication: Glaucoma/ocular hypertension
Mechanism of action ρ-Kinase inhibitor
Wholesale acquisition cost*: N/A
Macrilen (Small Molecule)
Active ingredient: Macimorelin acetate
Applicant: Aeterna Zentaris
Indication: Adult growth hormone deficiency
Mechanism of action Ghrelin mimetic
Wholesale acquisition cost*: N/A
Steglatro (Small Molecule)
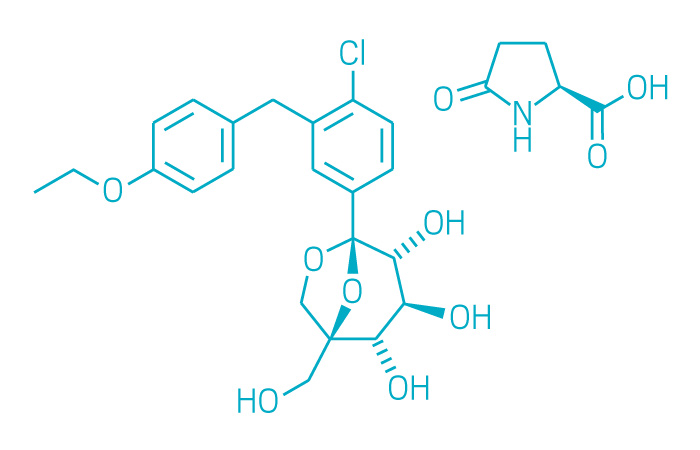
Active ingredient: Ertugliflozin
Applicant: Merck & Co./Pfizer
Indication: Type 2 diabetes
Mechanism of action SGLT2 inhibitor
Wholesale acquisition cost*: N/A
Giapreza (Peptide)

Credit: La Jolla Pharmaceutical
Active ingredient: Angiotensin II
Applicant: La Jolla Pharmaceutical
Indication: Hypotension in sepsis or critical illness
Mechanism of action Synthetic human angiotensin II
Wholesale acquisition cost*: N/A



















































Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter