Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Polymers
Strong, adhesive polymers made via stereochemical control
Using a chiral anion catalyst to prepare poly(vinyl ethers) transforms them from niche materials to practical plastics
by Bethany Halford
April 2, 2019
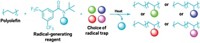
Taking a cue from chemistry that’s common in the pharmaceutical industry, chemists have used chiral anions to control stereochemistry when making poly(vinyl ethers). The resulting strong, adhesive polymers could find use in lightweight composites for making bicycles, boats, and cars, for example, according to Frank A. Leibfarth, a chemist at the University of North Carolina, Chapel Hill who spoke about the work Tuesday at the American Chemical Society national meeting in Orlando during a session in the Division of Polymer Chemistry.
Until now, poly(vinyl ethers) have been pretty niche products, Leibfarth said, because they’ve been atactic, meaning that the stereochemistry of the ether sidechains coming off the polymer backbone has been random. The resulting polymers are viscous liquids at room temperature and are primarily used as adhesives.

Leibfarth and postdoc Aaron J. Teator thought they could improve the properties of poly(vinyl ethers) by controlling the stereochemistry of the ether side chains so that they’re all identical, or, as polymer chemists say, isotactic. Leibfarth said he was inspired by the work of Nobel Prize-winning chemists Karl Ziegler and Giulio Natta, who developed a catalyst that produces isotactic polyolefins, such as polypropylene.
“This switch from atactic polypropylene to isotactic polypropylene essentially created a multibillion dollar industry because atactic polypropylene is almost useless,” Leibfarth said. But Ziegler-Natta catalysts become poisoned in the presence of Lewis basic heteroatoms like oxygen, which means they can’t be used to make oxygen-rich poly(vinyl ethers).
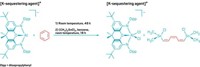
To control stereochemistry when making poly(vinyl ethers), Leibfarth and Teator turned to chiral anion catalysis, which has been used to control the stereochemistry of small molecules since 2000. The cationic polymerization takes place in a solvent with a low dielectric constant so that the cation at the end of the growing polymer chain tightly pairs with a chiral anion that dictates which side of the chain the next monomer will add to, resulting in an isotactic polymer (shown).

The isotactic poly(vinyl ethers) have excellent properties, Leibfarth said. They’re similar to commercial polyolefins but adhere more strongly to polar substrates, such as glass. Leibfarth and Teator also reported the work in Science at the end of March (DOI: 10.1126/science.aaw1703) and have filed a provisional patent application on the process (62/719,240).
Geoffrey W. Coates, a polymer chemist at Cornell University, called the work “a tour-de-force that combines mechanistic insight, synthesis, stereochemistry, and polymer properties.” He adds, “Given the relative lack of work in stereocontrolled cationic polymerization since it was originally reported by chemist Calvin Schildknecht over 70 years ago, it is great to have such an exciting advance that will revive research in this important area of polymer chemistry.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter