Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Microbiome
Cancer-related bacterial toxin induces latent viruses to start replicating
Findings hint at ecological role for colibactin
by Celia Henry Arnaud
March 1, 2022

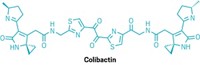
Certain Escherichia coli bacteria that live in the human gut produce the molecule colibactin, which binds to DNA and causes mutations associated with colon cancer. While this discovery provided a lead for treating or preventing colon cancer, scientists did not know why the microbes produce the molecule within the gut microbiome. In a new study, researchers report that colibactin induces replication of latent viruses in surrounding bacteria (Nature 2022, DOI: 10.1038/s41586-022-04444-3).
Justin E. Silpe, a postdoc in Emily P. Balskus’s lab at Harvard University, had previously worked with bacteriophages, viruses that infect cells and insert their DNA into bacterial genomes. There, the viral DNA can lurk until something triggers its expression and thereby the reproduction of the viruses. Silpe knew bacteriophage expression can be activated in response to DNA damage in the host. So when he and graduate student Joel W. H. Wong cultured bacteria that produce colibactin with bacteria that don’t and found that non-colibactin-producing bacteria developed DNA damage, the pair decided to test whether colibactin might be triggering bacteriophage activation in the damaged microbes.
The researchers infected bacteria with bacteriophages and then cultured the infected bacteria along with either colibactin-producing bacteria or non-colibactin-producing bacteria. After 24 h, cultures with colibactin-producing bacteria carried 100–1,000 times as many bacteriophages as did cultures without colibactin-producing bacteria. The colibactin-driven increase in bacteriophage populations also occurred when the bacteria were cultured with complex fecal microbiomes from mice.
“It’s still not clear whether [triggering bacteriophage production] is what colibactin is primarily evolved to do within microbiomes,” Balskus says. “What we can say is that production of colibactin within a microbiome causes DNA damage to other bacteria and in turn changes the viral component of the community.”
“For too long colibactin has been viewed with an overly anthropocentric approach, looking only at the impact of colibactin’s genotoxicity on the occurrence of cancer in humans,” Eric Oswald, a bacteriologist at Inserm, a French medical research institute, writes in an email. His team originally discovered colibactin in 2006. Oswald also says that the findings could help explain an observation from his lab that mice colonized at birth with colibactin-producing bacteria have different bacteria in their microbiomes than other mice do (mSphere 2020, DOI: 10.1128/mSphere.00589-20). The presence of bacteriophages could change the relative fitness of different species, resulting in these shifts in the microbiome, he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter