Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthetic Biology
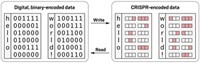
Digital information written directly into cells’ genomes using CRISPR
Engineered cells encode 0s and 1s as defined DNA sequences in response to voltage pulses
by Celia Henry Arnaud
January 14, 2021

Digital data are vulnerable to loss if the media they are stored on become obsolete. Floppy disks, anyone? In contrast, the ability to read biology’s storage medium—DNA—is unlikely to be lost as technology advances, making DNA of interest as a means of archival storage. Researchers have built a system designed to take digital information from a computer and record it directly into the DNA of bacterial cells. This approach eliminates the need for chemical synthesis of DNA, which has been required in previous methods for DNA data storage.
Before DNA can be used to store data, information must be converted from a digital to a biological format. Encoding digital data in DNA sequences usually requires translating the data into a DNA sequence that is then fed to a DNA sequencing machine to chemically synthesize the desired sequences. These are then stored as raw DNA, which is more susceptible to breakdown than DNA within cells. Researchers would like computers to talk directly to cells and for the cells to synthesize the DNA themselves in response to binary signals.
Harris H. Wang and coworkers at Columbia University have developed a method for such communication (Nat. Chem. Biol. 2021, DOI: 10.1038/s41589-020-00711-4).
Computers typically speak a language of electrons, encoded as voltage changes. To help cells translate this language, Wang and coworkers used a genetic construct originally engineered by William E. Bentley and coworkers at the University of Maryland, College Park (Nat. Commun. 2017, DOI: 10.1038/ncomms14030). The construct incorporates a redox-sensitive protein that bacterial cells use in response to oxidative stress in their environment. Bacterial cells with this construct sense an applied voltage, which induces them to make many copies of a circular piece of DNA called a plasmid. In the new system, Wang and coworkers combined that sensor with a system they previously engineered for inserting DNA into bacterial genomes (Science 2017, DOI: 10.1126/science.aao0958). Their system incorporates DNA from the electrically responsive plasmid into the bacteria’s genomes using enzymes that are part of the CRISPR gene editing system, which regularly inserts foreign and plasmid DNA into the bacterial genome as a sort of molecular memory. Usually the inserted sequence is random, but when voltage is applied, production of the plasmid is ramped up and the CRISPR system adds DNA from the plasmid to the genome.
“By capturing that [plasmid DNA], the cells have essentially recorded a bit of 1,” Harris says. “In the absence of this amplification, cells normally would just be recording 0s. This is how we’re able to distinguish 0s and 1s.”
In a demonstration of the approach, the researchers used their system to encode the message “Hello world!” So far the system takes several hours to write each bit, so the researchers used 24 bacterial populations in parallel to write the message, each recording 3 bits of data.
“The most important contribution of the work for data storage is the direct connection of electrochemical input signals with the recording of the data,” Albert Keung, a synthetic biologist at North Carolina State University who is working on DNA-based information storage systems, writes in an email. “This could lay the foundation for direct connections between traditional semiconductor-based systems with biological systems.”
“Wang and co-workers bring to the fore” many of the advantages of using living cells for reading, writing, and storing data. Cells can be propagated virtually indefinitely and at low cost, in parallel, and they can be sustained for “longish term memory,” Bentley, who was not part of the study, writes in an email.
Wang’s group is working on ways to speed up the data-writing process by evolving enzymes that work faster and more efficiently. To be able to write more data in parallel, they are shrinking the volumes needed for growing cell populations and increasing the number of populations they can handle at a time.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter