Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthetic Biology
Synthetic organelles modulate cell behavior
Compartments made from disordered proteins sequester and release enzymes
by Celia Henry Arnaud
August 6, 2021
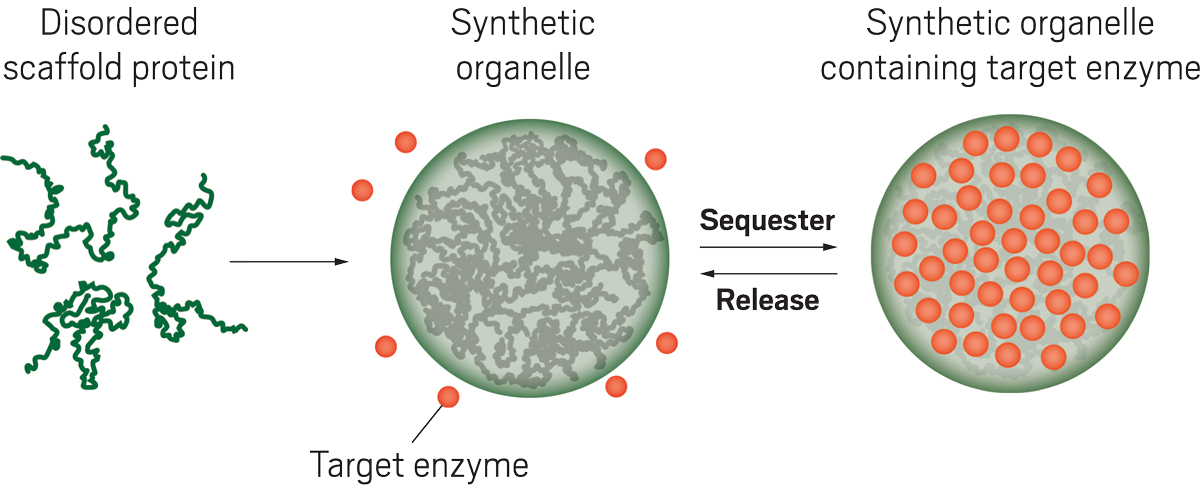
Cells use compartments known as organelles to sequester molecules or reactions as a way to control many biochemical processes. Scientists would like to do the same by engineering synthetic organelles into cells.
A team led by Matthew C. Good of the University of Pennsylvania programmed yeast to make synthetic organelles to sequester and release targeted enzymes in ways that altered the cells’ growth (Nat. Chem. Biol. 2021, DOI: 10.1038/s41589-021-00840-4). Researchers have made such synthetic organelles before, but they haven’t used them to program cell function.
That the researchers could turn cell growth off and back on makes this work “stand out from some of the previous work,” says Evan Spruijt, who studies artificial organelles at Radboud University and wasn’t involved with the new study.
Good and coworkers make their synthetic organelles out of a disordered scaffold protein that is known to form a separate liquid-phase droplet in solution. The researchers engineer yeast to produce both the scaffold protein and the enzyme that they want to capture within the organelle. They produce the protein and enzyme each attached to short protein sequences with high affinity for each other. This interaction has the effect of recruiting the enzymes to the synthetic organelle made by the scaffold protein.
For target enzymes, the researchers used enzymes that are part of the system that controls yeast cell growth. The synthetic organelles sequestered more than 80% of the target enzymes. The rate of cell growth dropped nearly 12-fold when the enzyme Cdc24, necessary for the type of yeast growth known as budding, was targeted to the synthetic organelles. Sequestering the enzyme Cdc5, required for another part of cell division, within organelles caused cells to stall midway through division, leaving them dumbbell shaped.
The functional changes can be reversed by linking the target enzymes to other short protein sequences that cause the organelles to release the sequestered enzymes in response to a stimulus such as temperature or light. For example, the researchers attached sequences to target enzymes that sequester the targets at room temperature but release them at elevated temperatures. Other sequences release the enzymes in response to light. After sequestered Cdc24 or Cdc5 enzymes are released from the synthetic organelles, the cells can once again divide normally.
The researchers also showed that the synthetic organelles can sequester targeted proteins in mammalian cells.
It’s hard to controllably and reversibly knock down the activity of an enzyme in its host organism by 80–90%, Good says, which is what sets the work apart from other synthetic organelle platforms that often are used for sequestering non-native proteins.
The work “demonstrates that designer organelles can indeed be used to control some very fundamental processes in cells,” Spruijt says. “This prospect obviously has significant potential, both for cell biology and synthetic cell research.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter