Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Food Science
Chemistry In Pictures
Chemistry in Pictures: Not-so-simple syrup
by Manny Morone
December 18, 2018

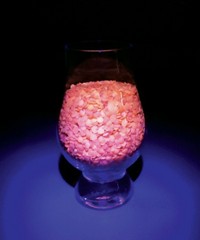
Although tasty, maple syrup seems chemically uninteresting. But, as part of his outreach theme of “#everydayfluorescence,” Brian Wagner posted this image of maple syrup glowing bright blue under an ultraviolet light. Wagner, a professor at the University of Prince Edward Island, found out about the sugary condiment’s glowing potential about two months ago when he read about it in a paper; he promptly went out to a grocery store to grab a bottle of President’s Choice maple syrup and has since tested other brands to confirm the result. According to Wagner, the fluorescence stems from the complex composition of the syrup, which includes various biological molecules from maple trees containing the amino acid tryptophan and phenolic moieties. Several of these aromatic molecules fluoresce.
Submitted by Brian Wagner via Instagram. You can send in your submissions via Twitter or Instagram by adding the tag #CENChemPics, and you can follow Wagner @fluorescent_chemist on Instagram and @DrummerBoy2112 on Twitter.
Do science. Take pictures. Win money. Enter our photo contest here.
Related C&EN Content:




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter