Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Green Chemistry
Scuba-diving spiders inspire carbon dioxide conversion
Gas-trapping coating on copper electrode boosts carbon dioxide reduction efficiency
by Prachi Patel, special to C&EN
August 21, 2019

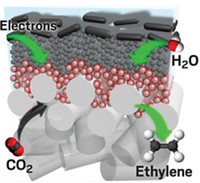
Inspired by a scuba-diving spider, researchers have made water-repelling copper electrode surfaces that boost carbon dioxide reduction by trapping the gas in bubbles (Nat. Mater. 2019, DOI: 10.1038/s41563-019-0445-x). This design converts five times more CO2 into the valuable chemicals ethylene and ethanol.
Electrochemical processes that use wind or solar power to convert carbon dioxide into hydrocarbons could be a win-win for the environment. The process would provide a use for CO2 captured from the air or other sources, provide a way to store intermittent renewable electricity, and offer an alternative source of chemicals that are otherwise made from petroleum.
Copper has proven the best CO2-reducing electrocatalyst so far. Researchers typically immerse copper-coated porous electrodes in an aqueous solution containing carbon dioxide. When electricity is applied, copper triggers the reduction of carbon dioxide into a mix of carbon monoxide and other products such as methane, ethylene and ethanol.
But copper has its drawbacks. The metal is not very selective, says Victor Mougel, a chemist at ETH Zürich. During catalysis, most of the electrons end up converting protons to hydrogen gas, instead of doing what chemists want them to do: reducing carbon dioxide. Researchers have tried to boost the efficiency of carbon dioxide reduction by nanostructuring the electrodes to increase their surface area or by doping copper with other elements.
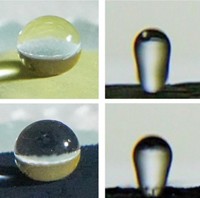
Mougel, Marc Fontecave of Collège de France, and their colleagues thought that trapping more carbon dioxide at the electrode surface would help the process. To do that, they turned to an arachnid for inspiration. The diving bell spider uses the hydrophobic hair on its abdomen to collect air bubbles that let it breathe underwater.
The researchers created a copper surface with tiny tree-like structures and coated it with a waxy layer of 1-octadecanethiol to make it hydrophobic. The nanoscale branches of the structures mimic the submicron dimensions of the spiders’ abdomen hair that allows them to form the gas bubbles. When immersed in a carbon dioxide-saturated aqueous electrolyte, the electrode trapped the gas as a large bubble at its surface, making more of it available for the catalytic reaction. This led to a significant performance boost. “This simple tweak drastically shifts the selectivity towards ethylene and ethanol with a drastic drop of hydrogen yield,” Fontecave says.
While the unmodified catalyst converts carbon dioxide to ethylene and ethanol with an efficiency of 9% and 4%, respectively, the hydrophobic catalyst boosts the yield to 56% and 17%. Hydrogen evolution, meanwhile, drops from 71% to 10%. The technique could also be applied to other catalysts for reactions such as nitrogen reduction to make ammonia, Mougel adds.
“This work demonstrates a new pathway to tune the selectivity, the most critical parameter in carbon dioxide reduction to fuels and chemicals,” says Cao Thang Dinh, a chemical engineer at Queen’s University in Ontario. By combining this strategy with other surface modification techniques, he believes the electrochemical conversion could be further increased.
However, the method is far from practical. The large gas layer makes less of the active catalyst surface area available for the reaction, which brings down the current and increases the voltage required, meaning making chemicals from CO2 using this process would take a lot of energy. The researchers plan to improve the process by fine-tuning the chemistry of the hydrophobic coating and experimenting with high-surface-area microporous electrodes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter