Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Persistent Pollutants
Evolved enzyme breaks silicon-carbon bond
Discovery points to a route for biodegradation of volatile methyl siloxanes
by Bethany Halford
January 29, 2024
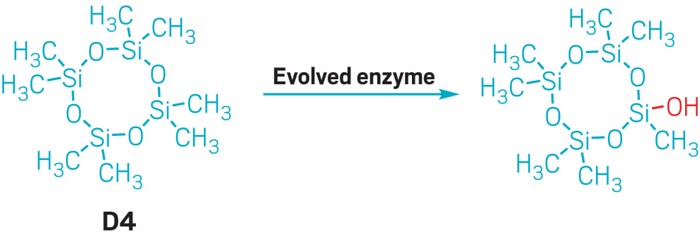
Using directed evolution, scientists have created the first enzyme known to break a silicon-carbon bond. The finding could be a preliminary step toward biodegrading volatile methyl siloxanes—chemicals that are made by the megaton each year.
Many consumer products contain volatile methyl siloxanes, including hair conditioners and lotions, and they’re also used to make larger silicone polymers. Some studies suggest these chemicals are persistent pollutants that accumulate in marine wildlife, including cormorants, seals, and turtles. The European Union has proposed restricting the use of cyclic methyl siloxanes known as D4, D5, and D6 and classifying them as persistent organic pollutants.
“Part of the reason we embarked on this research was the detrimental effects of siloxanes on biota as well as the environment,” says Nicholas Sarai, the study’s first author. He worked on the project when he was a graduate student in Frances Arnold ’s laboratory at Caltech.
Arnold is known for creating enzymes that can forge new bonds—work that garnered her a share of the 2018 Nobel Prize in Chemistry. In 2016, Arnold and coworkers created an enzyme that could create a Si–C bond. The work got the attention of scientists at Dow, a major producer of volatile methyl siloxanes, who reached out to Arnold to see if her lab could develop an enzyme that broke Si–C bonds. Arnold and Dow’s Dimitris Katsoulis led the latest project.
“There are no organosilicons in nature. And that’s why nature doesn’t break them down” using enzymes, Arnold says. “It’s just a bond that nature doesn’t really care about.”
The researchers found that a cytochrome P450 enzyme they had previously developed for breaking Si–H bonds could, to a small degree, also break a Si–C bond in volatile methyl siloxanes. Using directed evolution, they were able to improve the enzyme’s activity. The evolved enzyme catalyzes two tandem oxidations of a siloxane’s methyl group, which cleaves the Si–C bond. The transformation (shown) works on both cyclic and linear volatile methyl siloxanes (Science 2024, DOI: 10.1126/science.adi5554) .
“We’ve only been able to demonstrate that chemistry on one methyl group,” Sarai says. He notes that oxidizing a single Si–C bond in a volatile methyl siloxane does not biodegrade the molecule. “But it is a proof of concept that enzymes are able to do this silicon-carbon bond cleavage.”
“Our enzyme is terrible. It’s not a good enzyme, but it shows that it can be done,” Arnold says. “My whole goal is to open people’s minds about what biological systems can do. And this is such a great example of that.”
Ellie Browne, expert in siloxane environmental chemistry at the University of Colorado Boulder, says that there’s substantial interest in ways to degrade persistent siloxanes in the natural environment and in landfills. “Biodegradation is a potentially exciting and novel way forward,” she says in an email. But, she agrees that this is only a first step. “It would be exciting to see further degradation to break multiple Si–C bonds on a single siloxane,” she says.
Klaus Kümmerer, who studies sustainable chemistry at Leuphana University Lüneburg, says that the finding is an important step forward in siloxanes chemistry, but he thinks there are too many challenges with using evolved enzymes to degrade methyl siloxanes in the environment to make the approach viable. “We should design chemicals from the very beginning (benign by design) for fast and full environmental mineralisation by abundant natural enzymes,” he says in an email. Also, he says, “we should more think about whether certain chemicals, for example in cosmetics or shampoo, are really needed or whether they are just nice to have.”
UPDATE:
This article was updated on Feb. 28, 2024, to clarify Frances Arnold’s remarks about nature not breaking down organosilicons. She was referring to the breakdown via enzymes specifically.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter