Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
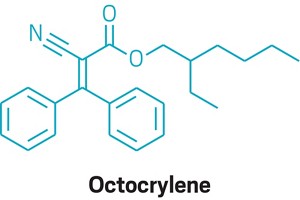
Common sunscreen ingredient octocrylene might be harmful to coral
High concentrations of octocrylene in seawater could impair fatty acid metabolism in coral
by Melissa Pandika
January 22, 2019

For the first time, researchers have shown that a common sunscreen ingredient, octocrylene, forms compounds that accumulate in coral and could impair coral metabolism at high concentrations (Anal. Chem. 2018, DOI: 10.1021/acs.analchem.8b04187).
In July 2018, Hawaii made history as the first state to ban the sale of sunscreens that contain oxybenzone or octinoxate, compounds some studies have shown could harm coral reefs. The new findings suggest other sunscreen ingredients may also warrant scrutiny and underscore the need to find alternatives that prevent skin cancer without harming aquatic life.
Didier Stien and colleagues at Sorbonne University and the French National Center for Scientific Research suspended pieces of Pocillopora damicronis coral in artificial seawater and treated them with different cosmetic ingredients at various concentrations. A week later, they prepared extracts from the coral pieces and determined the chemical structures of compounds in the extracts using fragmentation mass spectrometry. The team found the results of octocrylene treatment the most surprising.
The researchers detected 18 compounds consisting of octocrylene coupled with fatty acids. This coupling made the octocrylene more lipid soluble, enabling it to accumulate in coral tissues. They saw these compounds even at an octocrylene concentration of 5 μg/L, which is comparable to that in some aquatic environments, says Silvia Díaz-Cruz, an analytical chemist at the Spanish Council for Scientific Research not involved in the study.
The team did not see clear toxic effects at this concentration, at least over the one week of exposure in the study. But at 50 μg/L octocrylene and above, several fold higher concentrations than those detected in the environment so far, the researchers detected elevated levels of 16 acylcarnitines, metabolites that studies have correlated with malfunctions in mitochondria that impair the organelles’ ability to metabolize fatty acids.
They conclude that octocrylene is toxic at these levels and predict that it may also be toxic at environmentally relevant levels over longer exposure times.
Díaz-Cruz says other research groups, including her own, have detected octocrylene in dolphins, mussels, and other aquatic organisms, but the new findings mark the first to examine octocrylene metabolites. They also suggest these organisms are exposed to a higher environmental concentration of octocrylene than previously thought—accumulating in them not only in the form of octocrylene but also its fatty acid derivatives.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter