Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
2-D Materials
Introducing 2D semiconducting electrides
Discovery of unusual electron-rich compound may lead to applications in electronic devices and energy storage
by Mitch Jacoby
June 21, 2022

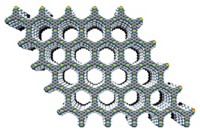
A group of exotic materials known for its abundance of electrons and exceptional electronic conductivity just welcomed an oddly behaved member to the family. This new member is a semiconductor, conducting electrons only under certain conditions (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.2c03024). The discovery of the first 2D electride that isn’t metallic may lead to applications in electronics and energy storage. The unexpected finding also shows that this quirky class of inorganic materials may have more tricks up its sleeve.
Electrides are unusual ionic solids often consisting of planes of atoms separated by sheets of delocalized electrons. As a result of these charged planes, electrides are highly conductive. All of the 2D layered ones reported until now are metallic.
So Lauren M. McRae, Scott C. Warren, and their coworkers at the University of North Carolina at Chapel Hill were surprised when the scandium-carbon compound they made turned out to be a semiconductor. “We didn’t know why it should be semiconducting,” Warren says, puzzled. “Shouldn’t these 2D electron planes act like superhighways for electrons?”
The team synthesized the compound as part of a study aimed at improving chemists’ ability to make new electrides and predict their behavior. Researchers who study these materials know that the components need to be electron rich and that the metal atoms that surround the electride sites must be electropositive, meaning they tend to give up electrons.
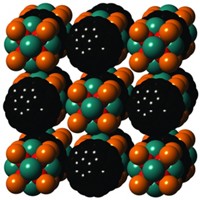
But those guidelines are too vague, Warren says. Many compounds meet those criteria but aren’t electrides. So the team systematically investigated the role of the metal’s electronegativity in a group of carbides. Specifically, they compared yttrium carbide (Y2C), which is known to be an electride, to two related materials that have yet to be reported or examined thoroughly: scandium carbide (Sc2C) and aluminum carbide (Al2C). The electronegativity values for yttrium, scandium, and aluminum are roughly 1.2, 1.4, and 1.6, respectively.
The researchers used high-temperature methods to prepare Sc2C and showed via X-ray methods, electron microscopy, and computational techniques that it is a layered electride. They investigated Al2C computationally.
Through their analysis, they realized that the interaction between electrons on the metal and those in the electride layer form a set of bonding and anti-bonding electron orbitals. The difference in the orbitals’ energies corresponds to the band gap, which is the energy difference between the material’s conducting and nonconducting state. Bandgaps are hallmarks of all semiconductors.
Greater electronegativity causes stronger electron interaction or orbital overlap, which leads to a larger bandgap. As a result, the carbide with the least electronegative metal, Y2C, has no bandgap—it’s metallic. Sc2C and Al2C are both semiconductors, but the aluminum compound has the larger bandgap as a result of aluminum’s greater electronegativity.
This study is “highly significant,” says Erin R. Johnson of Dalhousie University. She explains that the discovery of a semiconducting 2D electride means that these materials can exhibit a more diverse range of properties than previously thought, and that may open the door to new applications. Much of the work in this area is currently directed to exploring electride’s fundamental chemistry, Johnson says. But this class of materials has the potential to be used in many areas, including batteries, photodetectors, and semiconducting devices.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter