Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Holey films stand up to solvents and heat
Nanofilms made by combining techniques from interfacial polymerization and molecular layer deposition
by Bethany Halford
September 12, 2023
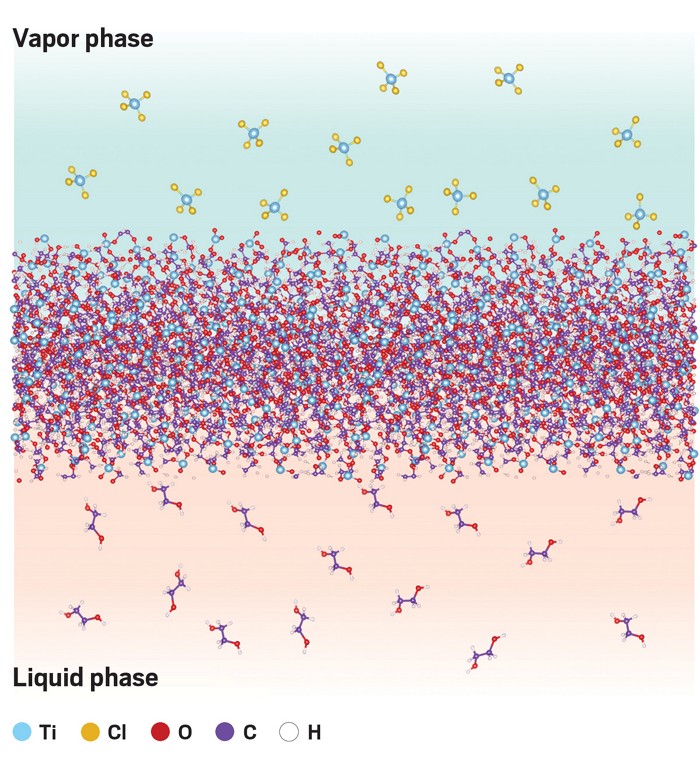
A new type of porous, inorganic membrane can separate compounds with molecular weights that range from 200 to 1,000. The material could be used to replace energy-intensive separations like distillation or crystallization when making pharmaceuticals or agrochemicals. The inorganic nanofilms are akin to polymer membranes used in desalination, but unlike polymer membranes, they won’t swell or degrade in organic solvents or at elevated temperature.
Scientists led by Miao Yu at University at Buffalo combined techniques from interfacial polymerization and molecular layer deposition to create the materials, which they call nanoporous carbon-doped metal oxide nanofilms. “The chemistry is not new,” Yu says. Rather, “we found a way to utilize what people have developed in different fields and engineered them together.”
The researchers expose liquid ethylene glycol to gaseous titanium tetrachloride, which combine to make a thin metal-oxide layer in a matter of minutes. They then heat this material to drive off some of the carbon, which produces holes in the film.
“The amount of carbon we leave behind is what determines the pore size of this membrane,” says Bratin Sengupta, a graduate student who helped lead the project. What’s more, because the membranes can withstand harsh organic solvents and temperatures up to 140 °C, they can be cleaned and therefore have a long lifespan.
For a proof-of-concept experiment, Yu’s team used two membranes—one with large pores and one with small pores—to separate the agrochemical boscalid from the catalyst and starting reagent that were used to make it (Science 2023, DOI: 10.1126/science.adh2404).
“This new method for making membranes enables the creation of mechanically robust and thermally stable materials that can operate in harsh conditions that sometimes are a challenge for more conventional membrane materials such as polymers,” says Ryan P. Lively in an email. Lively, an expert in energy-efficient separations at the Georgia Institute of Technology, says “there is a clear need to expand the operating window of these types of membranes to broaden the use of these energy-saving materials.”
Andrew G. Livingston, an expert in molecular separation membranes at Queen Mary University of London, calls the new membrane concept “an ingenious piece of work.” He says in an email that scale-up will be key to the membrane’s successful impact on industrial separations.
Yu says he hopes to start a company to develop and commercialize the inorganic nanofilm technology. He adds that there are many more chemical combinations to try with this process, with the potential to create films with a range of properties.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter