Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
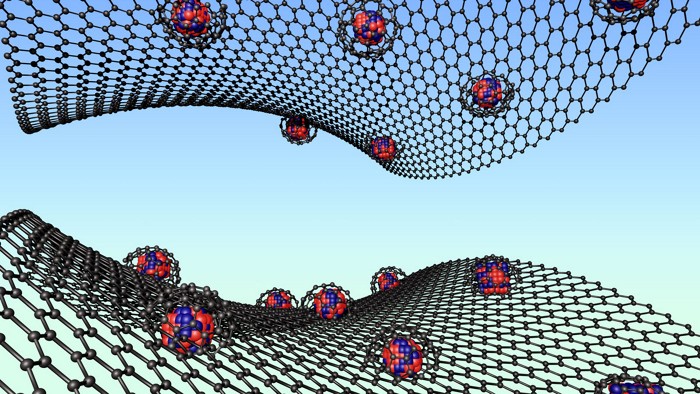
Metal-Organic Frameworks
MOFBOTS could carry drugs to specific targets in the body
Swimming corkscrew robots fitted with cargo-carrying crystals
by Mark Peplow
May 13, 2019

Like a shoal of miniature corkscrews, the MOFBOTS are coming. Spurred along by magnetic fields, these helical swimmers are the latest step toward building microscopic vehicles that carry therapeutic drugs to precisely where they’re needed in living tissue (Adv. Mater. 2019, DOI: 10.1002/adma.201901592).
The swimmers unite two well-developed technologies. Researchers have already created a range of torpedo-like nanorobots or microrobots that are propelled by chemical fuels, such as a stream of oxygen bubbles produced as the bot breaks down hydrogen peroxide. Other bots look more like motorized versions of cavatappi pasta, with a helical shape that can be steered around by a rotating magnetic field.
Meanwhile, metal-organic frameworks (MOFs) are showing promise as drug delivery systems. These crystalline lattices, made from metal-based nodes connected by organic struts, are extremely porous and can be loaded with medicines. But it has proved difficult to move MOFs around in a controlled way.
Enter the MOFBOTS. “We can equip a common microrobot with MOFs, and drive them under magnetic control towards a specific area,” says Josep Puigmartí-Luis at the Swiss Federal Institute of Technology in Zurich, part of the team behind the MOFBOTS. “This is the first example proving that these two fields can meet.”

The researchers used a 3-D printing technique to craft tiny helices, roughly 50 μm long, from a polymer resin. They coated the helices with nickel to make them magnetic, and titanium to make them biocompatible. Finally, they grew crystals of a widely used MOF called ZIF-8 on their surfaces, and loaded the MOF’s pores with a fluorescent molecule called rhodamine B, which acted as a proxy drug molecule. The researchers chose ZIF-8 because it degrades in mildly acidic conditions, such as those found around tumors.
Then the team used a weak magnetic field—less than the strength of a fridge magnet—to rotate the helical MOFBOTS, which corkscrew though water as they turn, similar to the tail-like flagellum that some bacteria use to move around. By varying the magnetic field, the researchers could steer the MOFBOTS along a pre-programmed course. “Combining magnetic propulsion with MOFs is a great step forward,” says Martin Pumera of the University of Chemistry and Technology, Prague, who works on nanorobots. “Magnetic navigation is very important for realistic applications.”
When the MOFBOTS were steered around a group of breast cancer cells in a petri dish, the acid environment broke apart the MOF crystals and freed their cargo of rhodamine B. Fluorescence imaging confirmed that the molecules were taken up by the cancer cells.
Pumera points out that drug molecules can also be attached to helical swimmers using organic linkers that are designed to release the drug once they meet a specific enzyme, for example. But Puigmartí-Luis says that MOFs have the advantage of protecting their cargo from the biological environment until they reach their intended destination. It’s also pretty easy to fine-tune the chemistry and structure of a MOF, so the carrier can be tailored to a specific drug molecule and the conditions it’s likely to encounter.
Other magnetic microrobots have already been steered around the eye of a live rabbit, and within the membrane that lines the abdominal cavity of a mouse. But deploying them in the bloodstream would be the toughest challenge, Pumera says. It might be possible to track swarms of bots using magnetic resonance imaging, but there is a risk they could block blood vessels, he says. One solution is to ensure the whole bot is biodegradable, so that the device gradually dissolves once its work is done.
Puigmartí-Luis agrees: “The next step is to make a fully degradable MOFBOT, and that’s what we’re working on now.”
CORRECTION:
This story was updated on May 16, 2019, to correct the length of the microrobots. They are roughly 50 μm long.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter