Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Photonics
Electrons hopping at the right speed are key to high-energy light emission
Certain materials give excited molecules enough time to align and upconvert photons
by Neil Savage, special to C&EN
March 26, 2024
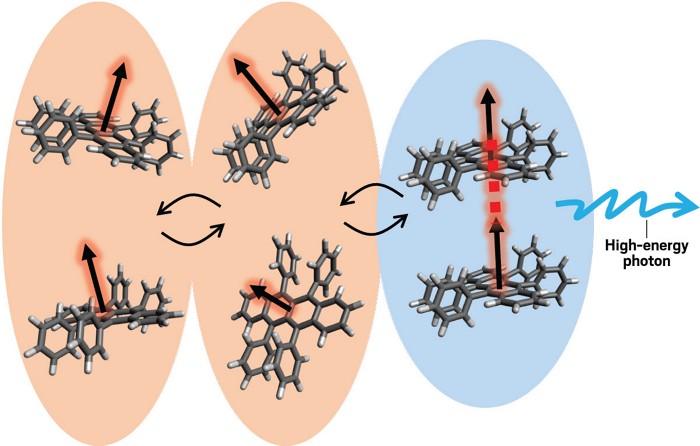
A better understanding of how electrons convert energy into photons could lead to designing more efficient solar cells or better phototherapies for cancer. Researchers looking into the process have figured out that the ease with which electrons can move through a material can make the material a more efficient converter (J. Phys. Chem. Lett. 2024, DOI: 10.1021/acs.jpclett.3c03602).
Under the right conditions, some molecules can supercharge light. After their electrons absorb a photon, they can take that energy and combine it with the energy of another similarly excited molecule, releasing a photon with twice as much energy as was in the original light. This so-called upconversion process can, for example, turn lower-energy infrared (IR) photons in sunlight into visible photons, which solar cells can convert into electricity.
In the new study, researchers from the Molecular Photoscience Research Center at Kobe University looked at how molecules in a triplet state—meaning the molecules were energized so that two unpaired electrons have the same spin—come together and trigger this upconversion. The key, they found, is having a material in which electrons can hop easily between molecules but do it slowly enough that they have time to align with another excited triplet molecule.
Under normal circumstances, only about 11% of triplet pairs combine in an orientation that’s just right and do the upconversion. So the team studied a special material system—a thin film of the hydrocarbon rubrene on top of a layer of a photosensitive thiophene compound—in which 77% of pairs emit light. The material’s light-emitting properties were known, but scientists did not understand why it was so efficient, says Yasuhiro Kobori, who led the research.
Using a technique called electron paramagnetic resonance, the researchers could track unpaired electrons as they tumbled around from molecule to molecule in the thin film. They found that for two molecules to combine and release a high-energy photon, the molecules need to align just right in a material and have about 1 ns to interact.
“Basically, if the hopping is too slow, there’s no encounter between triplet and triplet,” Kobori says, “but with too-quick hopping, we do not undergo the spin conversion.”
Understanding the behavior of triplets is the current bottleneck for understanding photon upconversion, says Ming Lee Teng, a professor of chemistry at the University of Utah, who was not involved in the research. But she adds that she’s “not sure about the practical possibilities because this result may only be applicable to disordered rubrene thin films.”
Kobori says this knowledge would allow scientists to create materials for more-effective solar cells. Another application could be phototherapy for cancer in which strong visible light is used to destroy harmful cells, he adds. Visible light doesn’t penetrate very deeply into the body, which has limited the development of this therapy. But doctors could potentially inject nanoparticles capable of upconversion into a patient, shoot the nanoparticles with more-penetrative IR light, and upconvert the IR light into higher-energy photons able to fight internal tumors.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter