Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nobel Prize
Podcast: Bioorthogonal, click chemistry clinch the Nobel Prize
Stereo Chemistry breaks down the science of prize winners Bertozzi, Sharpless and Meldal
by Ariana Remmel , Gina Vitale
October 5, 2022

The 2022 Nobel Prize in Chemistry was awarded to Carolyn Bertozzi, Morten Meldal and K. Barry Sharpless for their development of click and bioorthogonal chemistry which are used by chemists around the world to track biological processes and produce pharmaceuticals. In this special bonus episode of Stereo Chemistry, hosts Gina Vitale and Ariana Remmel delve into the science behind the prize and talk with organic chemist Antoni Riera to discuss the applications of the award-winning chemistry. C&EN contributor Mark Peplow also joins the Stereo Chemistry crew to talk about his conversation with Nobel Laureate Carolyn Bertozzi.
Read more about this award-winning science in Mark Peplow’s article about the 2022 Nobel Prize in Chemistry.
Music for this episode was “Rising Tide” by C.K. Martin. Press conference recordings were courtesy of ©The Nobel Foundation.
Subscribe to Stereo Chemistry now on Apple Podcasts, Spotify, or wherever you listen to podcasts.
The following is a transcript of the episode. Interviews have been edited for length and clarity.
Hans Ellegren: The Royal Swedish Academy of Sciences has this morning decided to award a 2022 Nobel Prize in Chemistry in equal shares to Carolyn Bertozzi, Stanford University California, USA, Morten Meldal, University of Copenhagen, Denmark, and to Barry Sharpless, Scripps Research, La Jolla, California, USA. They received the prize for the development of click chemistry and bioorthogonal chemistry.
Ariana Remmel: That’s right, it’s finally click chemistry!
Gina Vitale: That voice you just heard announcing the prize was Hans Ellegren, Secretary General of the Royal Swedish Academy of Sciences. He announced the prize in Stockholm on Oct. 5. The Stereo Chemistry team has been clicking away to bring you today’s bonus episode on this year’s prize-winning chemistry. We’ll start by going over what click chemistry and bioorthogonal chemistry are, and then we’ll hear from an organic chemist about the applications of that science. We’ll also talk to C&EN contributor Mark Peplow and hear some of his conversation with new Nobel laureate Carolyn Bertozzi.
Gina: I’m Gina Vitale, a life sciences reporter at C&EN.
Ari: And I’m Ari Remmel, a physical sciences reporter also at C&EN.
Gina: We’re your cohosts for today’s episode. So Ari, you were pretty excited about this click chemistry announcement. Can you tell me why?
Ari: So, for anyone who’s followed C&EN’s annual Nobel prediction webinar, click chemistry and bioorthogonal chemistry have been Nobel contenders for a while. Click chemistry describes a clever way to join two molecules by “clicking” two functional groups together. Antoni Riera is an organic chemist at the Institute for Research in Biomedicine, Barcelona. He describes it like this.
Antoni Riera: Click chemistry is a name that was coined by Barry Sharpless, like 20 years ago. And that means that these are a kind of reactions that are very easy to perform, very clean, and very fast. And these reactions are particularly useful for to bring two molecules together. So that’s why he coined the name of click chemistry, because you’re doing a click, you can join two fragments, like in a belt that you can do a click in, in an airplane belt.
Gina: So kind of like buckles on a backpack strap?
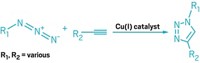
Ari: Yeah, exactly. People also sometimes use the analogy of LEGO blocks too. The idea is that the two molecules need to easily and selectively react with each other. Sharpless and Morten Meldal independently discovered that an azide and an alkyne can form a stable triazole ring with an assist from a copper catalyst. These functional groups turned out to be the perfect chemical partners to buckle together.
Gina: Now the name Barry Sharpless might sound familiar. He also won the Chem Nobel in 2001 for developing chiral catalysts for oxidation reactions. This award makes him only the second person in history to win a Chem Nobel twice.
Ari: Right. So Meldal and Sharpless found this reaction, which is now called the copper-catalyzed alkyne-azide cycloaddition, that snaps together these two chemical groups easily and only with each other. But copper is toxic to cells, so this first type of click chemistry wasn’t suitable for studying biological systems. That’s where Carolyn Bertozzi comes along and raises the stakes.
Gina: Basically, she figures out how to do these kinds of reactions without copper so she could introduce reactions into cells. And the reactions could mozy along without interfering with the cell’s biology; That’s where the term “bioorthogonal chemistry” comes from - orthogonal, meaning, at a right angle to something. This chemistry is effective, while still being at a right angle, so to speak, or not disrupting the biology.
Ari: So we’ve got a very versatile method of reacting chemicals here - you can join molecules quickly while avoiding unwanted byproducts, and even within cells.
Antoni Riera: This tool has been used in 1000s of experiments…mostly in biomedical science.
Gina: That’s Riera again. He uses click chemistry in his own research.
Antoni Riera: We have found that this is a reaction that is reliable, clean, and, of course, is the best way to join two molecules together … You can join an antibody with a small molecule, you can join a protein with a fluorophore, you can join a carbohydrate with a peptide. You can join whatever you can imagine. So the different possibilities are immense.
Ari: Whoa.
Gina: Right? Riera says that click chemistry has not only made some tricky biochemical syntheses easier, it also opened up whole new molecular architectures. And because azides, alkynes, and triazoles are chemical structures that aren’t found naturally in cells, these bonds aren’t easily broken apart by enzymes and other biological processes.
Ari: See? It’s all starting to click.
Gina: Oh my god.
Ari: Yes, well, one of our C&EN contributors, Mark Peplow, was able to reach prize winner Carolyn Bertozzi shortly after the prizes were announced to talk about the many applications of this science.
Gina: He’s with us today in the C&EN virtual studio. Welcome, Mark!
Mark Peplow: Hello!
Gina: How cool was it to get to talk to a newly minted Nobel laureate?
Mark: It was very cool indeed. Caroyln is always a pleasure to talk to. But on this occasion she was really quite busy, as you might expect. She said that her apartment was already full of Stanford press officers and cameras.
Carolyn Bertozzi: Wow, it’s like the red carpet treatment, you know!
Mark: Here’s what she told me about what it meant for her to win this year’s Nobel Prize in Chemistry alongside Sharpless and Meldal.
Carolyn Bertozzi: And I’ve known, you know, Barry Sharpless. He was a famous Nobel laureate when I was a grad student. And he came to Berkeley and gave a talk about enantioselective catalysis. And I was blown away. And then when I was in grad school, I read Morten Meldal’s papers on peptide synthesis and glycopeptide synthesis. So he, you know, was a legend in my mind as well, back when I was a student. So the fact that I’m now you know, in, my name is uttered in the same breath with those guys is, like, for me a huge honor.
Ari: Bioorthogonal and click chemistry have clearly found applications far beyond what any of the laureates could have imagined. Did she share anything about the broad reach of this field?
Mark: Absolutely. She told me that today’s award really feels like a recognition of the field as a whole. Like Riera was saying earlier, she said bioorthogonal chemistry and click chemistry have enabled more experiments than she could even count. But she said it’s been applied in two major ways, or buckets as she describes them.
Carolyn Bertozzi: The first bucket is using the chemistry as a discovery tool. So for example, a postdoc in my lab discovered the existence of glyco RNA using bioorthogonal chemistry, and it allowed him to see a whole new type of biomolecule.
Mark: The second bucket is drug development.
Carolyn Bertozzi: People in the biopharmaceutical industry are using these chemistries to discover what’s the target of their drug molecule and what are the off targets of their drug molecules. That’s a really popular application.
Gina: So are these methods actually being used to produce or deliver drugs?
Mark: Actually, yes they are!
Carolyn Bertozzi: There are now people who are actually doing bioorthogonal chemistry in human patients. And there’s one company I sit on the Scientific Advisory Board of, which is right now in a phase one clinical trial, where they’re injecting into patients two chemicals that react with each other in situ. And they’re using this to target chemo therapies to tumors more, more selectively.
Mark: We’ve been talking a lot about the biomedical applications of this technology, but it’s worth pointing out that click chemistry is now an integral tool for designing everything from new organic catalysts to next-generation polymers. In the press conference this morning, Bertozzi suggested that we have only seen the beginning of innovations that will likely continue to develop out of bioorthogonal and click chemistry.
Carolyn Bertozzi: I think the field of bioorthogonal and click chemistry is still I think in its early phases. And I think there’s probably many new reactions to be discovered and invented. So that’s an important frontier is inventing new chemistry. And then in terms of applications, again, I think applications in the biotech industry, the pharmaceutical industry, and the development of new ways of treating diseases and diagnosing diseases, I think that these are areas that will be very strongly impacted by click chemistry as they already have been.
Gina: Thank you, Mark for joining us on Stereo Chemistry! We’ve linked your story for C&EN in the show notes.That brings us to the end of our episode. It’s been a big day for the chemistry community and C&EN will continue to bring you news about this science as it clicks into place.
Ari: This episode of Stereo Chemistry was written by me, Ari Remmel, and Gina Vitale, and it was produced by Mark Feuer DiTusa. Story editing by Jessica Marshall. Production assistance by Krystal Vasquez and Mark Peplow. Stereo Chemistry’s executive producer is Kerri Jansen. Full credits for this episode are in the show notes.
Gina:Stereo Chemistry is the official podcast of Chemical & Engineering News. C&EN is an independent news outlet published by the American Chemical Society. Thanks for listening.
CORRECTION:
On Oct. 6, 2022, part of this episode was rerecorded and the transcript was updated to correct an error at 0:48 about when the 2022 Nobel Prize in Chemistry was announced. It was announced Oct. 5, not Oct. 6.
.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter