Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Antibiotics
Albomycin antibiotics discovered decades ago fall to total synthesis
Synthesis may guide analog production and inspire antibiotic strategies
by Celia Henry Arnaud
September 4, 2018

Sometimes the search for new antibiotics leads back to compounds that have been known for decades. Such is the case with albomycins, which scientists discovered in the 1940s. They were successfully used against human infections in tests in the Soviet Union in the 1950s but had not been further developed. Now, Yun He of Chongqing University and coworkers are reporting the first chemical synthesis for these soil bacteria-derived antibacterial compounds that could pave the way for next-generation antimicrobials (Nat. Commun. 2018, DOI: 10.1038/s41467-018-05821-1).
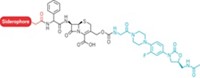
Albomycins are Trojan horse compounds composed of an iron-binding targeting region attached to a bacteria-inhibiting warhead region. The targeting region, called a siderophore, tricks the bacteria into taking up the warhead. In albomycins, a tetrapeptide siderophore joins forces with a thiosugar warhead. “The thiosugar portion is highly hydrophilic,” He says. “Alone it can’t cross the double-layered phospholipid membrane of bacteria and almost doesn’t have antibiotic activity,” but the siderophore boosts the thiosugar’s antibiotic activity by more than 10,000 times.
For the newly-reported synthesis, He and coworkers took a two-pronged approach. They generated the thiosugar portion and then attached it to the tetrapeptide. Making the thiosugar’s six consecutive chiral centers was the hardest part of the synthesis, He says.
He’s team produced three albomycins, δ1, δ2, and ε. In cell cultures, they tested the compounds against multiple strains of Streptococcus pneumoniae and Staphylococcus aureus, including three methicillin-resistant strains. Albomycin δ2 (shown) was consistently more potent than the other albomycins, as well as the established antibiotics ciprofloxacin, vancomycin, and penicillin. Albomycin ε was inactive against every bacterial strain the team tested.
Bharat Gadakh, a postdoc with Arthur Van Aerschot at KU Leuven, has been working on an albomycin total synthesis of his own. “Judicious choice of protecting groups for both fragments was the key to the successful synthesis, as we ourselves on many attempts have already experienced,” Gadakh says. Clever protection strategies allowed He’s team to achieve the desired stereoselectivity, both in the warhead portion and while connecting the two parts of the molecule, Gadakh says.
“While obviously not practical for commercial-scale synthesis, the chemistry will allow generation of analogs that might enhance structure-activity-relationship (SAR) studies that might guide bioengineered approaches to possible new antibiotics,” says Marvin J. Miller, who works on similar Trojan horse antibiotics at the University of Notre Dame. No compounds of this class are on the market yet, but a synthetic mimic is wending its way through clinical trials.
He’s group next plans to undertake such SAR studies to better understand how the thiosugar warhead inactivates bacteria as well as the transportation system that gets the molecules into bacteria.
“This paper on the synthesis of albomycins has significance beyond just being an impressive total synthesis,” Miller says. “It reemphasizes the need to consider natural bacterial defensive systems to help development of much-needed new antibiotics to keep us one step ahead in the never-ending microbial war.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter