Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Delivery
A gel for storing protein therapeutics
Material prevents proteins from aggregating, then releases them cleanly with the push of a syringe
by Bethany Halford
July 17, 2024

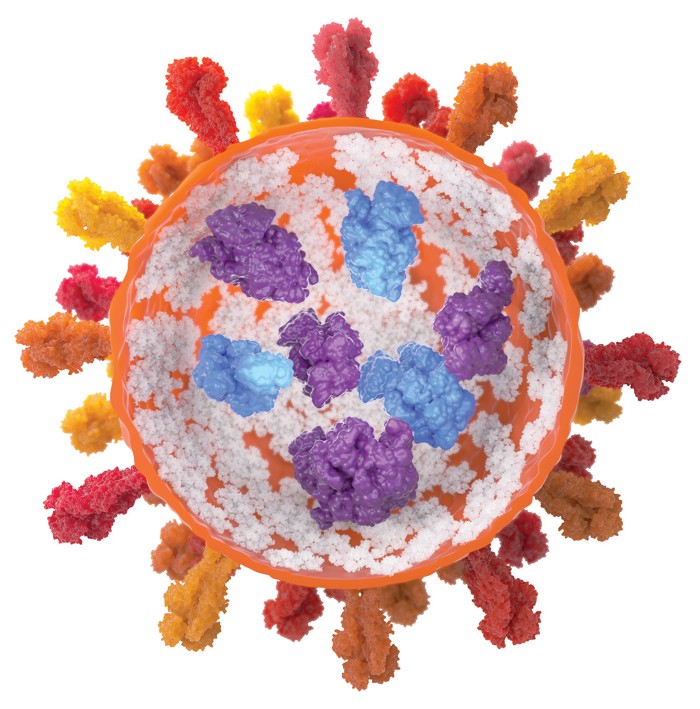
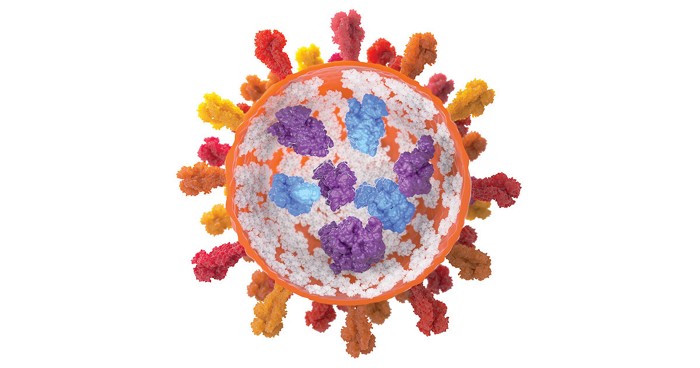
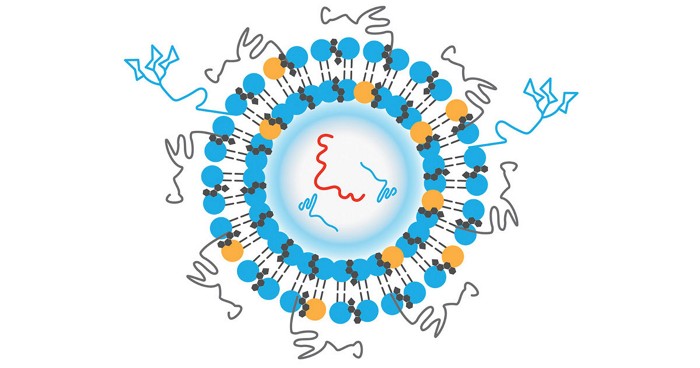
Protein therapeutics like insulin and GLP-1 inhibitors have a problem. They tend to aggregate when heated or shaken, robbing them of their potency. Researchers have now developed a low-molecular-weight supramolecular gel that prevents proteins from aggregating and then releases these drugs cleanly when injected a syringe fitted with a simple filter is used for injection. The finding could change the way protein therapeutics are formulated, possibly eliminating the need for cold storage (Nature 2024, DOI: 10.1038/s41586-024-07580-0).
Low-molecular-weight supramolecular gels tend to form stiff materials rather than squishy ones—something that’s been seen as a liability when they’re used for growing tissue or culturing cells. But scientists in Matthew I. Gibson’s group at the University of Manchester and Dave J. Adams’s group at the University of Glasgow reasoned they could use this perceived failing to their advantage.
If the protein-loaded gel is made within a syringe, the force of the syringe’s plunger causes the stiff gel to fail. It fractures and releases the protein and buffer. The gel’s fibers are much larger than the protein and get trapped within a filter at the end of the syringe.

“So, we have this wonderful mechanical release system, which is fantastically compatible with the practicalities of how a lot of therapies are delivered, which is in a syringe with a needle,” Gibson says.
The protein-loaded gels are easy to form within a syringe with the help of a gelator and a slight change in pH. The researchers demonstrated that the gel technology can store bovine insulin and β-galactosidase under conditions that would normally cause aggregation or deactivation.
Eric A. Appel, a professor at Stanford University who develops novel technologies for protein formulation and delivery, says in an email that the work is a “clever approach to solving a critical challenge with biopharmaceutical stability.” Appel says that while others have encapsulated protein drugs into hydrogel networks, those previous approaches left components of the hydrogel in the formulation when they were administered.
A crucial next step will be to demonstrate that the gels work across a broader range of formulation conditions, including a wider pH range, Appel says. “Regardless, this technology is exceptionally promising for improving the stability of current drugs to expand global access to critical therapies, as well as enabling new drugs from proteins that are simply not stable enough using traditional formulation approaches,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter