Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
Sanofi to acquire BTK inhibitor firm Principia for $3.7 billion
Principia is testing its small-molecule compounds in multiple sclerosis and immune system diseases
by Ryan Cross
August 18, 2020
Sanofi will pay $3.7 billion to acquire Principia Biopharma, a San Francisco-based biotech firm developing small molecules that inhibit Bruton tyrosine kinase (BTK). The price represents about a 75% premium over Principia’s stock market value in early July, before reports surfaced that Sanofi was interested in buying the firm.
BTK is a protein important for both normal B cell development and the proliferation of lymphomas, which are B cell cancers. AbbVie, AstraZeneca, and BeiGene all market BTK inhibitors for treating specific kinds of lymphomas. Sales of AbbVie’s inhibitor, Imbruvica, approached $4.7 billion in 2019.
Other drug firms have been eager to get in on the action as well. In January, Merck & Co. spent $2.7 billion to acquire ArQule, whose experimental noncovalent BTK inhibitor is designed to overcome resistance that some cancers develop after treatment with current covalent BTK inhibitors. Eli Lilly and Company’s $8 billion acquisition of Loxo Oncology in 2019 also included a noncovalent BTK inhibitor.
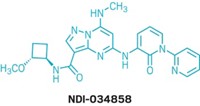
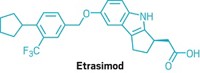
BTK is also linked to inflammation, and Principia focuses on developing BTK inhibitors for immune system diseases and multiple sclerosis. Its compound rilzabrutinib is currently in clinical trials for pemphigus and immune thrombocytopenia. In 2017, Sanofi struck a deal to develop Principia’s brain-penetrant BTK inhibitor, SAR442168, for multiple sclerosis.
Sanofi announced in April of this year that the inhibitor reduced formation of new lesions—the scarred nervous tissue that gives multiple sclerosis its name—by 85% in a Phase II clinical trial. A Phase III trial of the compound began in June.
Upon announcing its deal to acquire Principia, Sanofi said that both rilzabrutinib and SAR442168 have the potential to become a “pipeline in a product,” indicating they can be used for many immune-related and neurological diseases, respectively.
The anti-inflammatory effects of BTK inhibitors have raised interest in the drugs as treatments for people hospitalized with COVID-19. Notably, the US National Cancer Institute conducted a small study suggesting acalabrutinib may help reduce the respiratory distress and inflammation in people with COVID-19. Based on that preliminary study, AstraZeneca—which markets acalabrutinib as Calquence—is conducting a 60-person randomized trial of the drug for COVID-19.
Sanofi has not indicated interest in investigating Principia’s BTK inhibitors as COVID-19 treatments.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter