Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Discovery
Small molecule prevents rogue bone formation
The molecule could treat an extremely rare disease
by Sarah Braner
June 7, 2024
A new small molecule could treat and prevent excess bone formation.

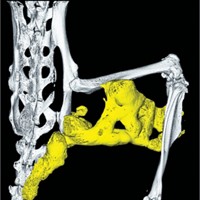
Fibrodysplasia ossificans progressiva (FOP) is an extremely rare disease estimated to affect about one in 2 million people worldwide. People with the condition experience heterotopic ossification, in which bone replaces skeletal muscle and connective tissue.
“Usually by adolescence, children’s necks are involved, their shoulders are involved, and they can’t extend their elbows, they can’t reach over their heads; in fact, they maybe can’t raise their arms at all. And later in life, during the second, third decade, heterotopic bone forms in the jaw and in the lower limbs. Eventually, they essentially form a second skeleton,” says Frederick Kaplan, an expert in the condition at Penn Medicine who was not involved in the new research.
The condition complicates the healing process after an injury too. Normally, when someone is bruised or has a minor injury like a needle stick for a routine vaccination, the injury heals by replacing the damaged tissue. Instead, in people with FOP, bone forms at the injury site, causing pain and further movement problems.
In a new paper published May 29 (Sci. Transl. Med. 2024, DOI: 10.1126/scitranslmed.abp8334), researchers from Blueprint Medicines detailed the discovery and development of a molecule called BLU-782 as a potential treatment. It inhibits the ALK2 protein, a widespread cellular receptor that is especially prominent on muscle, tendon, fascia, and ligament cells. In its mutated, overactive state, BLU-782 starts a process that instructs cells to create extra bone tissue where it doesn’t belong.
Using a mouse model of FOP, the researchers showed that administering BLU-782 could prevent heterotopic ossification after an injury like a muscle pinch or a broken bone. While it was most effective if daily treatment was started before an injury, the treatment prevented ossification if started 2 days after the injury but was less effective at 4 days. Researchers saw the best results if the injured mouse had been treated with BLU-782 before the injury and continued to be treated for at least 12 days after.
Additionally, the team had to administer the drug daily for it to work because the base problem of the faulty receptor hadn’t been solved. The drug just blocks its effects, so when the drug isn’t present, the receptor continues to cause problems.
“It’s like having a light bulb that never turns off in your bedroom, and you just cover it up with a sheet. . . . The second that the sheet gets pulled away, the light’s back on,” says Timothy LaBranche, one of the authors of the new paper and the vice president of preclinical safety at Blueprint Medicines.
One unknown factor is whether this treatment is suitable for children. “The extra skeletal bone formation in FOP can be quite expansive during childhood, and it’s critically important to try to prevent that bone formation during those early years,” says Eileen Shore, another expert in the condition at Penn Medicine who was not involved with the new paper.
The only currently FDA-approved drug for FOP, called Sohonos and distributed by Ipsen, is approved only for those aged 8 years and older for females and 10 years and older for males. Sohonos blocks formation of heterotopic bone, but it also blocks new bone tissue from forming at the epiphyseal growth plate at the end of long bones. This isn’t as much of a problem for adults, but it is for children who need to grow, according to Shore. It’s unknown whether BLU-782 will cause similar problems.
While this is the first time this data has been published to a wide audience, the molecule has already gone through Phase 1 clinical trials, and Ipsen—who has acquired the licensing rights and renamed it fidrisertib—is currently conducting Phase 2 trials.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter