Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
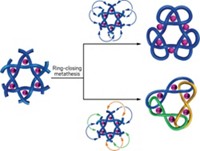
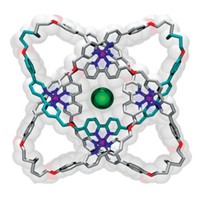
Chemists tie a knot with 12 crossings
The 378-atom molecule wraps three knots together in a central knot to create a trefoil of trefoils
by Bethany Halford
March 11, 2022

Sailors, take note: chemists may soon claim the title of makers of the most complex knots. The latest molecular loop-de-loop to come from David A. Leigh’s lab at the University of Manchester features 378 atoms and a record-breaking 12 crossings. The knot that previously held the record had 9 crossings. The new knot is a trefoil of trefoils triskelion—a three-way crossing of three-way crossings that features three limbs radiating from its center (in case you’re not up on your knot lingo). The chemists also prepared an isomer of the knot that switches up the structure of the central trefoil (Science 2022, DOI: 10.1126/science.abm9247).
“The most important feature of the work is not really the complexity of the knot, but rather that it’s such a large, extended, essentially 2D array of well-defined crossings,” says Leigh in an email. He points out that the knot consists of one continuous strand, making it a knitting project of sorts. “If you think about it knitting is an amazing process in which a 1D strand is entangled to form a 2D layer that, if done correctly, can adopt a pretty persistent and functional 3D shape” like socks or a sweater, he says.
To create the knot, Leigh’s group used Vernier templating, which takes advantage of mismatching the numbers of coordination or recognition sites on molecular building blocks to create large complexes. The whole project took 3 years to complete. Zoe Ashbridge, the PhD student who Leigh says “made it all happen,” created the knot within a few months. “But finding the right methods for the purification was extremely tricky as was obtaining all the necessary structural proofs, for example demonstrating that the crossings were what we intended,” he says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter