Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Consumer Safety
US FDA finalizes hand sanitizer rule
Agency seeks additional data on 3 active ingredients, bans 28 others
by Britt Erickson
April 11, 2019

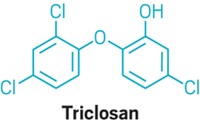
Manufacturers can no longer use 28 active ingredients, including triclosan and benzethonium chloride, in over-the-counter hand sanitizers sold in the US, under a rule finalized by the US Food and Drug Administration on April 11. Millions of consumers rely on hand sanitizers to reduce bacteria on their hands when soap and water are not available.
The FDA stopped short, however, of banning the use of three active ingredients—benzalkonium chloride, ethyl alcohol, and isopropyl alcohol—in hand-sanitizer products. Instead, the agency will continue to seek additional safety and effectiveness data for those three chemicals to determine whether they are generally recognized as safe and effective for use in consumer hand sanitizers.
“We believe industry has made good progress toward providing data and we will continue to provide updates to the public about the progress of collecting this data,” Janet Woodcock, director of the FDA’s Center for Drug Evaluation and Research, says in a statement, referring to the three chemicals that the FDA deferred from further regulation.
The FDA proposed the rule in 2016 and requested data on the three ingredients at that time. Manufacturers are welcoming the additional time to generate the data.
“Consumers can continue to use hand sanitizer products with confidence as this regulatory process moves forward. We will work to ensure that these products remain available,” Richard Sedlak, executive vice president of technical and international affairs at the American Cleaning Institute, which represents the US cleaning products industry, says in a statement.
The FDA predicts that banning the 28 chemicals will affect less than 3% of the US hand-sanitizer market. Most consumer hand sanitizers sold in the US contain ethyl alcohol, according to the FDA. Retailers have stopped selling hand sanitizers that contain triclosan, but a small number of products still contain benzethonium chloride, the agency says. Manufacturers who wish to continue making hand sanitizers that contain any of the 28 chemicals will need to have their products approved as new drugs by the FDA before they can be legally sold in the US.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter