Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Automated synthesis creates longest polysaccharide to date
Achievement paves the way to a better understanding of carbohydrate structures and functions
by Fernando Gomollón-Bel, special to C&EN
May 22, 2020

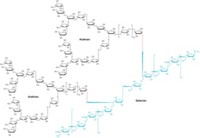
Polysaccharides—long chains of simple sugars—play central roles in biological systems, including supporting cell structure, storing chemical energy, and regulating cell recognition. But synthesizing polysaccharides in the lab is a herculean task. Now, using automated methods, a team of researchers has prepared the longest polysaccharide ever synthesized and has done it in record time. The team made a 100-unit-long sugar polymer in less than 200 hours, achieving an overall yield of 8% (J. Am. Chem. Soc. 2020, DOI: 10.1021/jacs.0c00751). The researchers also made a branched polymer with 151 sugars, demonstrating that their automated approach can create more complicated structures.
“The game has been officially changed,” glycochemistry expert Carolyn Bertozzi of Stanford University says in a tweet.
For a long time, chemists tried to make long sugar chains using classic organic synthesis strategies. “Polysaccharides have been more challenging to synthesize [than other biopolymers] because they have far more diverse linkage structures,” Bertozzi explains to C&EN. Polysaccharides can have many branches and stereocenters, so researchers synthesizing them need to consider “variations in regiochemistry and stereochemistry for each monomer connection,” she says. Preparing even a short oligosaccharide this way could take several weeks. Indeed, the previous largest synthetic carbohydrate was a 92-unit molecule constructed by joining together shorter blocks, an accomplishment that took more than 2 years (Nat. Commun. 2017, DOI: 10.1038/ncomms14851).
In 2001, Peter H. Seeberger of the Max Planck Institute of Colloids and Interfaces started adapting automated solid-phase methods, which until then had only been used to make peptides and nucleic acids, to prepare polysaccharides. In this process, molecules are covalently attached to a resin with a linker. Then the molecule is grown step by step using a series of glycosylation reactions. After the polysaccharides reach the desired length, they are cleaved off the resin and purified. An automated machine carries out the many cycles of reactions needed to yield the final product.
Seeberger and his colleagues spent 2 decades optimizing the process, boosting the efficiency of every step. “We really pushed the limits to make reactions as fast and high-yielding as possible,” he explains. “We have studied every single bottleneck.” This included finding a linker to the solid-phase resin that can withstand hundreds of coupling reactions but yet is easy to cleave once the molecules are finished. The researchers also had to improve purification and mass spectrometry techniques so that they could accurately characterize the extremely long polysaccharides as they were being made.
The ability to make synthetic polysaccharides would help enable scientists “to understand their biological functions in normal and pathological settings,” Bertozzi says. Seeberger is confident that, with minor tweaks, the machine could soon synthesize even longer polysaccharides, and in much shorter times. The current methodology only uses one type of sugar unit—mannose. “Given that there are likely millions of unique polysaccharide structures in nature...the next steps will surely be to generate structures with more diverse monomer constituents,” Bertozzi says.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter