Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Electrochemistry forges C–C bonds in two new cross-electrophile couplings
Reactions show off electrochemistry’s scope
by Bethany Halford
March 1, 2022
Chemists are amped up about two new reactions that use electricity to form different types of carbon-carbon bonds. One transformation builds sp3-sp3 C–C bonds and the other creates sp2-sp3 C–C bonds. Adding these transformations to the synthetic chemist’s toolbox should spark interest in electrochemistry, an increasingly popular molecule-building tool, experts say.
Electrochemical transformations use electricity rather than chemical reagents to perform reactions. Both of the new reactions are cross-electrophile couplings, which wed two different electrophiles. Although there ways to do cross-electrophile couplings using chemical reagents, these alternatives are often plagued by homocouplings, where one electrophile forms a bond with another electrophile of the same kind instead of with the second electrophile.
Shannon Stahl, an expert in organic synthesis at the University of Wisconsin–Madison who was not involved in the research, says in an email that “both of these studies show that organic electrochemistry is evolving to a point that it’s positioned to challenge conventional catalytic methods for bond construction.”
In one reaction, chemists led by Cornell University’s Song Lin and California Institute of Technology’s Kimberly A. See discovered they could use electrochemistry to form bonds between alkyl halides in an electrochemical version of the classic SN2 reaction (Nature 2022, DOI: 10.1038/s41586-022-04540-4).
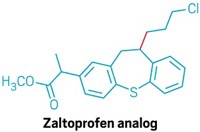
“Our idea was really to think about how electrochemistry can provide new reactivities,” Lin says. Chemists can dial in electrochemical potentials that allow them to differentiate between two different alkyl halide electrophiles, he explains. Injecting electrons turns the more substituted electrophile into a nucleophile, which attacks the less substituted electrophile to make a new bond. The team used this reaction to combine myriad electrophiles to create compounds including an analog of the nonsteroidal anti-inflammatory drug zaltoprofen (shown, new bond in red).

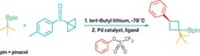
The other cross-electrophile coupling reaction, from a team led by Phil S. Baran of Scripps Research, California, Héctor D. Abruña of Cornell University, and Scott L. Anderson of the University of Utah, builds terpenes and polyenes (example shown, new bonds in red) in a modular manner by coupling vinyl halides with redox-active esters (Science 2022, DOI: 10.1126/science.abn1395).

The key to getting this nickel-catalyzed reaction to work, Baran says, was to add silver nitrate. It’s not an intuitive addition: the reaction is a reduction and silver nitrate is an oxidant. But the silver nitrate deposits silver nanoparticles onto the electrodes, which fine-tunes their reactivity. “The silver nanoparticle effect is a general one,” Baran says, “and one that I would bet will find extensive use in modern pharmaceutical chemistry.”
Daniel J. Weix, an expert on cross-couplings at the University of Wisconsin–Madison, says in an email that “these two breakthroughs show how combining electrochemistry with cross-electrophile coupling can enable synthetic disconnections and allow for a degree of control not possible with simple chemical reductants.” This work, he says, builds on other examples to make “a convincing case that electrochemistry tools should be standard equipment in any synthetic laboratory.”
UPDATE
This story was updated on March 2, 2022, to clarify that researchers specify, or "dial in," particular electrochemical potentials that allow them to differentiate how two alkyl halide electrophiles react during a cross-coupling reaction. Because of an editing error, an earlier version implied that researchers distinguished the electrophiles by moving closer to target potentials.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter