Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Photochemistry unleashes a one-two radical punch for efficient ring synthesis
Iridium and nickel catalysts cooperate to install nonaromatic rings in drug molecules
by Mark Peplow, special to C&EN
March 18, 2024
A light-driven reaction sequence that bolts nonaromatic rings onto heteroarenes, such as pyridines, offers a rapid route to medicinal molecules that would be troublesome to make using existing methods (Nature 2024, DOI: 10.1038/s41586-024-07181-x).
Adding saturated sp3 carbons into drug candidates can improve properties such as solubility and target-binding affinity. But fusing nonaromatic rings to heteroarenes can be laborious, leaving this type of structure underrepresented in medicinal chemistry compound libraries.

A team led by David W. C. MacMillan at Princeton University has now developed a one-pot reaction system that creates these fused ring systems using simple and readily-available radical precursors such as diols and bromoalcohols. Collaborators at Janssen Research and Development are already using it in their medicinal chemistry work.
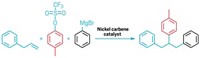
The process initially uses blue light and an iridium photocatalyst to generate a carbon radical from the precursor. A nickel catalyst then helps that radical to couple to the heteroarene. MacMillan’s group reported this strategy in 2021, but the new system now adds a second coupling to the mix.
After the precursor has been tethered to the arene, the iridium removes its other functional group to generate another carbon radical. This locks on to an adjacent point on the heteroarene to close up a nonaromatic ring.
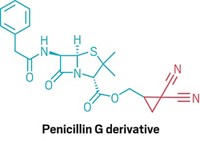
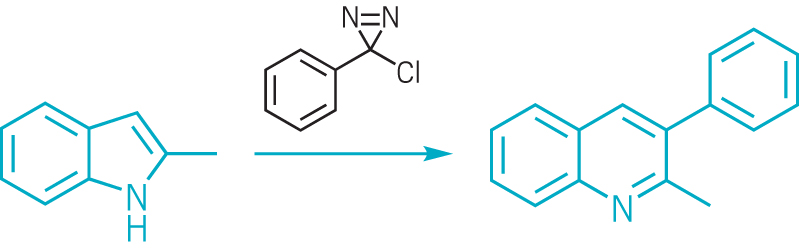
The team showcased more than 50 examples with a range of heteroarenes—including pyridines, quinolines, and pyrimidines—and created analogues of drugs such as the cystic fibrosis treatment Lumacaftor (shown). The reaction does not interfere with other functional groups in the molecules, so medicinal chemists can use it to modify complex molecules in the final stages of a synthesis. “We were surprised how well-behaved the whole thing was,” MacMillan says.
Advertisement
He adds that the reaction could be applied to a much wider range of coupling partners than diols or bromoalcohols. “You can make radicals from almost anything now,” MacMillan says. “So this strategy should work with almost any two functional group precursors that you want to use.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter