Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
How to add a carbonyl to a molecule using carbon monoxide and light
Palladium-catalyzed reaction makes acid chlorides, amides, esters, and ketones from both alkyl and aryl halides
by Bethany Halford
April 16, 2020
| A version of this story appeared in
Volume 98, Issue 15

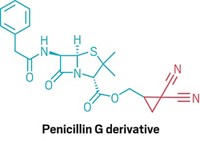
Carbonyls (C=O groups) are popular reactive handles. But carbonylation reactions, which create these useful groups by appending carbon monoxide to aryl halides or alkyl halides with the help of a metal catalyst, tend to work with only a few types of molecules. So even though it’s possible to make ibuprofen on an industrial scale using carbonylation, the reaction isn’t a common tool.
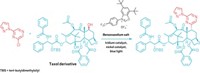
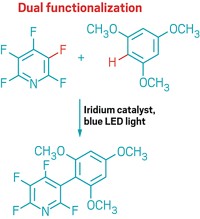
But that could change, thanks to a discovery by McGill University chemists Bruce A. Arndtsen, Gerardo M. Torres, and Yi Liu. These researchers found that by shining blue light on a palladium-catalyzed carbonylation, they could make the reaction work with many types of aryl halides and alkyl halides. The transformation can be used to make acid chlorides, amides (example shown), esters, and ketones, motifs found in or en route to a large number of pharmaceuticals (Science 2020, DOI: 10.1126/science.aba5901).
Ilhyong Ryu, a chemist at Osaka Prefecture University who studies catalysis, says this reaction is “amazing” because it is a simple system that works with many different substrates. “It’s like carbonylation in automatic mode without the need to change gears,” he says. “It is also striking to see that acid chlorides can be synthesized by their system. Acid chlorides have been a difficult class of carbonyl compounds to prepare by carbonylation chemistry.”
Arndtsen says the group discovered the reaction while trying to use photoredox catalysis to make acid chlorides via carbonylation. “When we started looking more closely, we realized the photoredox catalyst was doing nothing.” Instead, he says, the blue light itself was doing the work, by exciting two stages of the catalytic cycle—a radical-induced oxidative addition and a radical-induced reductive elimination.
“Catalysis is a lot about balancing,” Arndtsen explains. “Anything you do to help one side of the catalytic cycle oftentimes hurts the other side.” But the blue light helps drive both sides, striking a balance that’s otherwise difficult, if not impossible. “By using light to drive both, you can start to do hard steps in both directions, and that’s what adds a lot of the generality here.”
Vladimir Gevorgyan, a chemist at the University of Texas at Dallas who has used light to spur palladium-catalyzed oxidative addition reactions, says that the McGill group’s reaction is “a highly useful method for mild and general carbonylative functionalization of organic halides, dramatically broadening the boundaries of this important methodology.” He adds that the approach could also be used with other cross-coupling reactions.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter