Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
An up-close view of catalysis in real time
Scientists observe how different temperatures alter interactions between metal nanoparticles and support material
by Neil Savage, special to C&EN
May 22, 2023

One way to fight global warming is to capture carbon dioxide before it enters the atmosphere and convert it into something useful, such as methane, which can be a fuel or a chemical feedstock. That conversion, which bonds hydrogen atoms to carbon atoms, requires a catalyst, and now a group of researchers has uncovered how a certain class of catalysts can be treated to make them more efficient (Science 2023, DOI: 10.1126/science.adf6984).
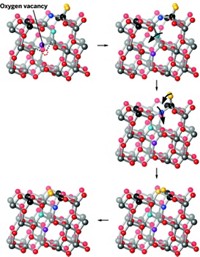
Scientists have long known that placing a metal catalyst atop a different material could improve catalytic performance, a phenomenon called strong metal–support interaction (SMSI). The new study marks the first time researchers have actually seen in real time what’s going on in SMSI and how it can be adjusted for optimum performance.
The team set nickel nanoparticles atop titanium dioxide and used a combination of electron microscopy and vibrational spectroscopy to examine how individual atoms behaved when the catalyst was preheated and then exposed to CO2 and hydrogen. When they heated the catalyst to 400 °C, the TiO2 formed thin overlayers coating the nanoparticles. When they subsequently introduced the gases, the overlayers crawled off the nickel, leaving it completely exposed. With pretreatment to 600 °C, however, the titanium only slid off certain facets of the nanoparticle during catalysis, leaving the nickel only partially exposed. That left behind more interface sites between the titanium and the nickel where carbon reactions could take place, which made the catalysis more active and selective, working at a faster rate and creating fewer unwanted byproducts.
Bert Weckhuysen, a chemistry professor at Utrecht University in the Netherlands and one of the leaders of the work, says other temperatures probably lead to different amounts of interfaces between the materials, and he plans to study those to see if they give even better results. He also hopes to look at other SMSI systems, such as cobalt on titania.
Redesigning the nanoparticles to create different facets to which the overlayers cling might also provide a way to fine-tune the catalysis, says Sara Bals, a professor of physics at the University of Antwerp in Belgium and another leader of the study. “This is a completely new playground, and we look forward to exploring that new playground,” she says.
Wenyu Huang, a professor of chemistry at Iowa State University, calls the study “a substantial advancement” in the field. “This work will profoundly impact catalyst design for many important reactions that require harsh reaction conditions,” he says.
And Ding Ma, a professor of chemistry at Peking University in China not involved in the study, says the results are a “groundbreaking finding” in SMSI, which “has been the hot topic in catalysis.” He says the knowledge provided by the research “could be surely used to design new efficient/effective catalyst systems.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter