Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
CO₂ converts into formaldehyde at room temperature via a shelf-stable intermediate
The method can also be used to incorporate isotopic labels into organic molecules to aid drug discovery
by Cici Zhang, special to C&EN
November 13, 2019

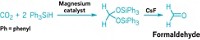
Carbon dioxide is abundant and renewable, so chemists would like to find more opportunities to use the gas in organic synthesis. One potential approach is to turn it into formaldehyde, a precursor for many industrially produced chemicals. But this conversion is not easy, requiring high temperatures and expensive catalysts. Now, researchers have designed a method to produce formaldehyde from CO2 by creating an intermediate compound that is shelf stable and quickly releases formaldehyde upon treatment at room temperature (J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.9b08342). What’s more, the method makes it easier to do isotopic labeling of organic molecules, which has applications in drug discovery.
The current methods for making formaldehyde from CO2 involve a sequence of reactions at elevated temperatures catalyzed by a precious metal. A few years ago, while looking for nonprecious metal catalysts for CO2 conversion, Gerard Parkin of Columbia University and colleagues discovered a zinc catalyst that reduces CO2 by helping to add a Si–H across the molecule to produce a silyl formate (J. Am. Chem. Soc. 2012, DOI: 10.1021/ja308500s).
In the new study, the researchers use a magnesium-based catalyst to produce a bis(silyl)acetal, H2C(OSiPh3)2, from CO2 and triphenylsilane, Ph3SiH. The compound remained stable and didn’t decompose, even after being stored in a vial for 60 days. By adding cesium fluoride to the compound at room temperature, “we could activate the molecule and convert it to formaldehyde instantaneously,” Parkin says.
In the lab, formaldehyde is often made by cracking paraformaldehyde—a commercially available material—at high temperatures. The advantage of the Columbia team’s method is that “you have a solid that is easy to handle and releases formaldehyde under mild conditions,” says Oliver Trapp of the Ludwig Maximilian University of Munich, who, along with his colleagues, developed a way to turn CO2 into a formaldehyde derivative using ruthenium, a precious-metal catalyst (J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.8b10233).
Clifford P. Kubiak, a chemist of the University of California, San Diego, who specializes in CO2 conversion, says the global chemical industry uses only about 115 million metric tons of CO2 each year as a feedstock, mostly for urea synthesis. So using more to make formaldehyde would be “a good thing,” he says. But manufacturing chemicals from CO2 would only use up 0.5% of global emissions, so it’s unlikely to reduce CO2 accumulation in the atmosphere, he adds.
Kubiak says “the real value” of Parkin’s bis(silyl)acetal approach is the ability to prepare isotopically labeled formaldehyde equivalents and related molecules.
To demonstrate, the Columbia researchers used 13C-labeled CO2 and deuterium to make methyl acrylate via the bis(silyl)acetal. In the future, chemists could also use the method to mark drug candidates with labeled CO2 and labeled bis(silyl)acetal. Isotopic labeling of a drug candidate would allow chemists to track and evaluate its properties once it enters the human body by seeing where it goes and how it is metabolized.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter