Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Electron beams take aim at pharmaceuticals
Drug developers make use of electron diffraction’s potential to unlock tiny crystals’ mysteries
by Bethany Halford
October 17, 2022
| A version of this story appeared in
Volume 100, Issue 37
Back in 2010, crystallographer Tim Gruene heard a scientist from drugmaker Novartis say something that stuck with him. The pharmaceutical giant had amassed a collection of around 2 million small molecules, but only about 10% of those could be analyzed using X-ray crystallography. This special 10% was amenable to assembling into large, high-quality crystals that crystallographers could bounce X-rays through. The angles and intensities at which those beams exited the crystal could be decoded to reveal the molecules’ structures. This method is the gold standard for figuring out what a molecule looks like in three dimensions, allowing researchers to glean exactly how the molecule’s atoms are connected to one another.
Another one-third or so of the molecules were also crystalline, the Novartis scientist said, but they were powders, which meant that the crystals were too small for single-crystal X-ray analysis—they simply didn’t give enough data when hit with X-rays. While chemists have other ways of determining how a molecule is connected, such as nuclear magnetic resonance spectroscopy and mass spectrometry, these methods deliver connectivity by inference. If the data are ambiguous, chemists can get the structure wrong.
Incorrect structures are particularly troublesome for pharmaceutical candidates. Misassign the structure of a small molecule when you first make it, and you could lose time and money trying to make that same, wrong structure using a different chemical route. Incorrect structures could also lead scientists to misinterpret how the molecule is binding in its active site, and could therefore misdirect efforts to improve a compound’s biological properties.

If scientists could somehow assemble the same 3D pictures that they got from X-ray analysis using a technique that worked on smaller crystals, it would help them pin down precisely what the other third of the molecules in their collection were. Gruene recalled all of this when he started a new job studying proteins a few years later. His new lab used electron diffraction (also called 3D ED or microED—which term to use is somewhat controversial), a technique that works like X-ray diffraction but uses data gathered from how electrons, instead of X-rays, diffract from a crystal. Because electrons interact with molecules more strongly than X-rays do, it’s possible to get data from crystals that are much smaller—as small as 100 nm—as opposed to the micrometer-sized crystals needed for X-ray analysis.
Thinking he could use electron diffraction to unlock the structural secrets of powders like those in Novartis’s library, Gruene decided to switch his focus from proteins, which could be notoriously difficult to prepare for electron diffraction experiments, to small molecules, particularly pharmaceuticals. Although Gruene was unaware of it at the time, a handful of research groups had been developing electron diffraction methods to analyze small molecules for decades. But the technique hadn’t caught on with organic and medicinal chemists or with pharmaceutical makers.
Hosea M. Nelson, an organic chemist at the California Institute of Technology who uses electron diffraction, says that even though scientists had been using the technique to study small molecules since at least the 1990s, the people guiding that field “were looking for perfection,” he says. They wanted a technique that could give precise bond lengths and angles. But most chemists studying organic molecules, such as natural products and drugs, aren’t looking for that. What’s most useful for these scientists about electron diffraction is that it tells them about connectivity, or how the atoms are hooked together.
Nelson uses automotive engineers as an analogy. Early electron diffraction researchers “were trying to make Formula 1 race cars that go 300 mi per hour,” he says. “There’s a whole group of customers that just need something that’ll take them to the grocery store at 20 mi per hour. And they weren’t talking to each other.”
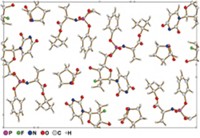
That began to change in October 2018 when Gruene, who is now head of the Center for Chemical Structure Analysis at the University of Vienna, led a group that showed how to tailor an electron microscope so that its diffractometer could be used to solve the structures of small organic molecules. To demonstrate this technique’s applicability to pharmaceuticals, the researchers broke open a capsule of cold medicine and used electron diffraction to solve the structure of the acetaminophen powder inside (Angew. Chem., Int. Ed. 2018, DOI: 10.1002/anie.201811318).
A day after Gruene’s paper appeared online, a team, including Nelson, from Caltech and the University of California, Los Angeles, reported on the preprint server ChemRxiv that it was similarly able to use electron diffraction to solve the structures of several small organic molecules, including drugs and natural products. The researchers also analyzed a mixture of four compounds all on the same small sample collector. “You could actually tease out the structures of all four molecules directly from that mixture” using electron diffraction, says UCLA’s Tamir Gonen, who was one of the lead authors on the report. “I’m actually not aware of any other structural biology method that is able to deliver atomic-resolution structures directly from mixtures.” A peer-reviewed paper of this work appeared in ACS Central Science the following month (2018, DOI: 10.1021/acscentsci.8b00760).
Those two publications made organic and medicinal chemists take notice, and since then, the technique has been growing in popularity. Electron diffraction is now poised to make major inroads into pharmaceutical chemistry and other areas where chemists want structural information about small organic molecules.
“We have already shown the field this technique is very important and useful,” says Xiaodong Zou, an electron diffraction expert at Stockholm University. Now, she says, chemists who make drugs need to adopt it. “They will find that because of this technique, they can do drug development in a very different way than what they usually do.” They can take advantage of the technique to explore new strategies for making specific polymorphs—different crystalline forms a small molecule can take—and cocrystals, she says.
Electron diffraction shouldn’t necessarily be the first structural elucidation tool to turn to, says Jessica Bruhn, who works on electron diffraction at the specialized contract research organization NanoImaging Services. “If you already have large crystals, you should definitely do single-crystal X-ray diffraction. The data quality is really high and the equipment is much cheaper. It’s just a very well-established field.” But electron diffraction shines, she says, when you have very little material or when you’ve run into problems growing large crystals.
Because electron diffraction analysis works on tiny crystals, scientists can use the technique when they have vanishingly small amounts of material. Natural product research—in which chemists painstakingly extract molecules from plants, bacteria, fungi, or other organisms—is “an area where the quantity of material and the size of the crystals is a limiting factor in structural elucidation,” Caltech’s Nelson says. A chemist might have to start with hundreds of kilograms of a natural product’s source to extract just a few hundred milligrams of material needed for structural analysis using X-ray crystallography and NMR spectroscopy. But Nelson says he’s been able to use electron diffraction methods to solve the structures of complex molecules with just nanograms of material.
For medicinal chemists, electron diffraction is a tool they can use to visualize the compounds they make, which takes any guesswork out of structural elucidation. The technique also helps process chemists because it can identify polymorphs. Polymorphs of the same active pharmaceutical ingredient (API) can possess properties that differ, including melting point, solubility, and chemical stability.

Electron diffraction can give scientists a better idea than single-crystal X-ray diffraction of what polymorphs are present in an API that’s being manufactured on large scale, according to Grahame Woollam, a scientist at Novartis who uses the technique. With X-ray crystallography, he says, “you have a crystallographer that picks out one nice looking crystal that really might not be representative of your material at all.” With electron diffraction, he says, you simply pour the material you want to study onto a transmission electron microscope grid and stick it in the instrument. “So it’s very representative of the material that you make from your crystallization conditions on your scale.”
Researchers at Stockholm University and Sun Yat-sen University recently used electron diffraction to resolve what they describe as a “47-year-old misunderstanding” of polymorphs of the nonsteroidal anti-inflammatory drug indomethacin. Indomethacin has been on the market since 1965. In 1974, chemists found a previously unidentified polymorph of the drug, which they said could result from either melt or solution crystallization. The team from Stockholm and Sun Yat-sen found that these crystallization methods led to two distinct polymorphs, one of which was entirely new. The finding could have implications for the drug’s formulation (Angew. Chem., Int. Ed. 2021, DOI: 10.1002/anie.202114985).
Another advantage of electron diffraction is its ability to quickly scan the thousands of crystals in a sample for impurities and find out what they are. This identification is useful for quality control and fraud detection, Woollam says. “You literally take a capsule, you empty the contents onto the grid, and you’re able to analyze this.”
Electron diffraction also reveals the locations of light atoms, including hydrogen and lithium—something that X-ray crystallography can’t do. Woollam says that information is important for those seeking regulatory approval or patent protection because it gives a more accurate picture of drugs. Chemists can see hydrogen atoms that are connected to nitrogen and oxygen atoms as well as protons in salts that are frequently used when formulating drugs.
The technique’s precision even extends to stereochemistry. Though researchers didn’t used to think it was possible, Lukáš Palatinus, a crystallographer at the Czech Academy of Sciences, and his colleagues recently showed they could assign a molecule’s stereochemical arrangement using electron diffraction. Assigning stereochemistry required a change to how the data were processed, not any changes to the experiments, according to Palatinus. Palatinus was part of a team that used electron diffraction to solve the structure—including the stereochemical configuration—of the hepatitis C treatment sofosbuvir in a cocrystal with
Practical advances are drawing more scientists to electron diffraction, Palatinus says. “They are adopting the method because they see the results.” A few instrument makers have taken notice. Palatinus points out that Thermo Fisher Scientific now sells software that makes it easier to turn an electron microscope into an electron diffractometer, and two instrument makers—Eldico Scientific and Rigaku—are now selling stand-alone electron diffractometers.
“Electron diffraction has been done mostly in research labs and in the academic context using transmission electron microscopes, which requires a lot of knowledge in terms of electron microscopy,” says Petra Simoncic, Eldico’s chief innovation officer. Eldico was founded to make a stand-alone electron diffractometer, known as the ED-1. A benefit of the instrument, Simoncic says, is that it doesn’t require knowledge of how to adjust an electron microscope. Researchers just need to prepare their samples and place them in the instrument. Once the samples are inside, she says, you simply hop from crystal to crystal collecting data, which takes about a minute for each crystal.
Mark Benson, who leads sales and marketing for Rigaku’s stand-alone electron diffraction instrument, known as the XtaLAB Synergy-ED, says the instrument has had an enthusiastic reception. “I’ve been at Rigaku for 17 years, and it’s the most groundbreaking development I’ve ever seen.”
Some researchers say the instruments are expensive. Benson wouldn’t give a precise price but conceded that “it’s not insignificant.” Simoncic estimates that Eldico’s ED-1 costs between $1.5 million and $1.8 million.
“I do think that there is a fundamental flaw in what those companies are doing because they’re charging way too much for those machines,” UCLA’s Gonen says. “For the amount of money that they’re asking, I could get an electron microscope from other companies that can do everything.”
The instrument makers disagree. A new transmission electron microscope with all the latest features is more expensive, Simoncic says, and no specialized personnel is required to run Eldico’s stand-alone diffractometer, which reduces maintenance costs. Benson says the instruments have already demonstrated their value. Even though Rigaku’s instrument has been available for only a little over a year, users are already publishing results from their experiments with the instrument. For those who don’t want to invest in their own electron diffractometer, both companies also offer contract services in which researchers can send their samples for electron diffraction analysis.
Advertisement
Cost isn’t the only drawback of electron diffraction as an analytical technique. Robert Bücker, Rigaku’s product manager for electron diffraction, says that because electrons can propagate only in a vacuum, all samples must be able to withstand vacuum conditions. This can be challenging for molecules that are crystallized as hydrates or contain large solvent channels, Bücker says, but there are ways to address this challenge—usually by freezing samples.
And not everyone who has used electron diffraction is a fan of the technique yet. Mohammad R. Seyedsayamdost, who studies natural products at Princeton University, recently used electron diffraction to determine the structures of algae-killing molecules produced by bacteria that live on algae (Angew. Chem., Int. Ed. 2021, DOI: 10.1002/anie.202114022). But he points out that even though the method works with extremely small amounts of material, that material still must be crystalline. “You can’t improve the fact that you need crystals. It is just an inherent limitation of the method, and that’s not going to change,” he says. Seyedsayamdost estimates that only a small fraction of the natural products his group works with can be crystallized.
Caltech’s Nelson, who has been an advocate for electron diffraction in recent years, agrees that the technique has limitations. The ability to analyze extremely small crystals is what makes electron diffraction attractive, but it can also be a drawback, he says. Those small crystals might not be representative of the whole sample. There have been times, Nelson says, when the chemists in his lab have made a mistake in identifying a material “not because we got the structure wrong—it’s because we actually saw the structure of an impurity or of a very minor and irrelevant component of the sample,” he says.
Even with these drawbacks, chemists say the technique has a place in the analytical tool kit, although whether it will be a mainstay or a niche technique still remains to be seen. The University of Vienna’s Gruene says he has already contacted a few drugmakers, large and small, and is helping them use electron diffraction to elucidate the structures of their compound collections—tapping into that once-mysterious third.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter