Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Electron crystallography could be a powerful tool for organic chemists
Two teams demonstrate the visualization of small molecules with electron-bombarding technique
by Bethany Halford
October 23, 2018
| A version of this story appeared in
Volume 96, Issue 43

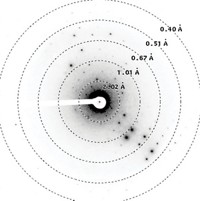
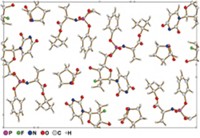
When chemists want to determine the structure of a molecule, they typically turn to X-ray crystallography. But chemists often find they can’t grow the large, high-quality crystals required for analysis. Now, a similar technique, known as electron crystallography, which works with smaller crystals, is poised to become an alternative. Two teams working independently show the method quickly determines the structures of small organic compounds, offering organic chemists the ability to analyze a wider array of small molecules than they can with X-ray crystallography.
Electron crystallography is similar to X-ray crystallography, but scientists study the diffraction pattern made by firing electrons at a crystal rather than X-rays. Electrons interact more strongly with the molecules in crystals than X-rays do, which means researchers can use vanishingly small amounts of material—crystals as small as 100 nm. Crystals studied with X-ray crystallography typically need to be at least 5 µm in all dimensions.
Scientists haven’t used electron crystallography to study organic molecules regularly because the electron beam tends to destroy the crystals before enough data can be collected. But in recent years, researchers have managed to modify the technique so that it can be used to study delicate biomolecules, such as proteins. They cool samples down to cryogenic temperatures and use an attenuated electron beam.
Two teams found they could apply similar modifications to analyze tiny crystals of organic compounds. Tim Gruene, a scientist at the Paul Scherrer Institute, spearheaded a research effort based in Europe (Angew. Chem. Int. Ed. 2018, DOI: 10.1002/anie.201811318). A U.S.-based team was led by Jose A. Rodriguez, Hosea M. Nelson, and Tamir Gonen of the University of California, Los Angeles, along with California Institute of Technology’s Brian M. Stoltz (ChemRxiv2018, DOI: 10.26434/chemrxiv.7215332.v1, with publication pending in ACS Cent. Sci.).
Using modified electron crystallography techniques to study organic molecules isn’t new, both Gruene and Gonen point out. Both researchers have previously published results from such techniques in crystallography and molecular biology journals. But those reports went largely unnoticed by the chemistry community.
In the new reports, both teams highlight the technique for organic chemists by showing that they can crack open a capsule or grind up a tablet of over-the-counter medicine, such as the painkiller acetaminophen, and use the technique to determine the structure of the active pharmaceutical ingredient. They also apply electron crystallography to larger organic compounds, such as a methylene blue derivative, in the case of the European team, and the antibiotic thiostrepton, in the case of the U.S. team. A rough analysis of some powders could be done in as little as 20 minutes, Nelson says, which is similar to simple X-ray crystallography experiments.
“To see an over-the-counter cold and flu medicine capsule being cracked open and the heterogeneous powder inside analyzed at atomic-level resolution is awesome,” says Tom Maimone, an organic chemist at the University of California, Berkeley. “Even if this technique only works for a subset of organic small molecules, what is shown in these papers is stunning.”
Advertisement
The technique does have limitations. While the analysis requires only a small amount of material, that material must be crystalline. And at the moment, the technique can only determine a molecule’s relative stereochemistry, not its absolute stereochemistry.
Even so, the researchers expect this technique will be popular among organic chemists and other researchers interested in small molecules, provided they can gain access to the instruments they need. Nelson points out that UCLA has only one cryo-electron microscope for performing electron crystallography, and it’s mainly used by the school’s biologists. Gruene says his instrument is really a prototype, with a sophisticated detector connected to an electron microscope. Both Nelson and Gruene hope by showing what this technique can do, more instrument makers will design electron microscopes with organic chemists in mind.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter