Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Imaging
New PET probes light up bacteria in deep-seated infections
Microbe-specific tracers could help detect infection without relying on inflammation as a surrogate
by Jyoti Madhusoodanan, special to C&EN
March 12, 2020

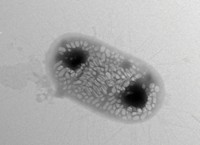
Infectious bacteria that burrow into bones, lungs, or other deep-seated tissues can be tough to detect. Clinicians often rely on positron emission tomography (PET) scans, where an injected radio-labeled tracer reveals evidence of disease. But existing PET tracers detect the body’s response to infection—not the pathogens themselves—which can make it hard for clinicians to know if a bacterial infection has resolved. Now, researchers have developed a new radiotracer that marks live bacteria in living animals, which could help clinicians determine whether a patient needs antibiotics (ACS Cent. Sci. 2020, DOI: 10.1021/acscentsci.9b00743).
Molecular probes like those used in PET imaging are being developed to visualize cancer cells, tissue damage in rheumatoid arthritis, concussions, or other inflammation-causing conditions. “But not many new tracers are available to study infectious disease,” says Alvaro Ordoñez, who studies imaging of infectious diseases at Johns Hopkins University and was not involved with the study. “Any new tools are great, and this work is a great advance for the field.”
To create a PET probe specific to bacteria, David Wilson of the University of California, San Francisco and his colleagues turned to D-alanine, an amino acid found in bacterial cell walls but not in mammalian cells. They synthesized radiolabeled versions of D-alanine using 11C, and found that bacteria grown in flasks incorporated the labeled amino acids into their cell walls.
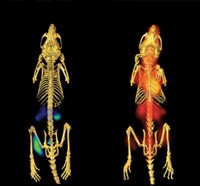
The researchers created shoulder injuries in mice to create inflammation, then injected live bacteria into the animals’ right shoulders and heat-killed microbes into their left. They injected the 11C-labeled D-alanine probe into the animals’ bloodstream and performed PET scans on the animals. The areas with living pathogens produced a signal 3.5 times as high as those with dead microbes, revealing that the probes were specific to bacterial infection, not just inflammation. When they tested other PET probes such as a commonly used form of labeled glucose or gallium citrate that detect inflammation, both shoulders yielded the same signal.
Inflammation doesn’t always mean bacteria are present; it can also result when mammalian cells respond to injury, the presence of tumor cells, or other causes unrelated to bacteria, says Emory University’s Kiyoko Takemiya, who studies imaging methods to detect cardiac implant infections and was not involved with this study. “It’d be great to have a probe that can detect active infections instead of just spotting all inflammation,” Takemiya says.
In another experiment, the team repeated the shoulder injuries, then injected antibiotic-resistant Escherichia coli into one joint and antibiotic-sensitive strains into the other. They found that after antibiotic treatment, the D-alanine tracer’s signal was completely lost on the side with antibiotic-sensitive bacteria, suggesting the technique could monitor the effectiveness of drugs. “To see that signal decrease after antibiotic treatment, that’s huge,” Ordoñez says.
Mammalian cells don’t use D-alanine, but they can absorb the amino acid and convert it to a different, usable version, or digest it to pyruvate, a molecule that’s used by nearly all living organisms. A probe that the team tested using two bound D-alanine molecules may circumvent this issue. Wilson says the team is working on other ways to prevent the probe being redirected through these routes. Future studies will also need to confirm the minimum number of bacteria that can be detected by these tracers, Takemiya adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter