Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Ultrasound-bouncing bubbles help track bacteria in mice
Bacteria engineered with gas vesicles allow researchers to use ultrasound to image the microbes at higher resolutions and greater depths than before
by Stu Borman
January 4, 2018
| A version of this story appeared in
Volume 96, Issue 2

Some scientists have engineered bacteria to target tumors to deliver cancer drugs or to travel to the gut to fight off pathogenic strains. To improve such possible microbial treatments, researchers would like to know where the engineered microbes actually go in the body. Currently, the most popular method is to get bacteria to express fluorescent proteins and then track the microbes through their bioluminescence. But bioluminescence-based techniques work at depths of only a few millimeters below the body’s surface, and their resolution degrades quickly with depth.
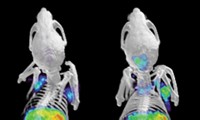
Mikhail G. Shapiro of Caltech and coworkers have now developed a new technique based on ultrasound imaging that improves imaging depth to more than 10 cm and substantially enhances spatial resolution (Nature 2018, DOI: 10.1038/nature25021).
The inspiration for the technique came from photosynthetic microorganisms that produce protein nanostructures filled with dissolved cellular gases to help them float, allowing the cells to soak up sun at the water’s surface. The Caltech team discovered that these bubbles also bounce sound waves back to a source. Ultrasound imaging works by detecting sound waves reflected by objects inside the body.
The researchers therefore designed a set of genes from different photosynthetic bacteria and archaea that would allow other bacteria like Escherichia coli to produce their own gas bubbles. These bubbles would allow scientists to use ultrasound imaging to spot the microbes in the body.
When the researchers introduced the genes into E. coli, the bacteria produced about 100 sound-bouncing vesicles per cell. The vesicles trap a mixture of gases present in the cells, such as oxygen, nitrogen, and carbon dioxide. Slightly changing one of the genes leads to bubbles that respond to sound waves in distinct ways. The researchers exploited such a variation to use ultrasound imaging to distinguish two types of bacteria simultaneously.
Reporter genes for ultrasound is a totally unexpected concept that nobody else in the ultrasound field envisioned before, says Olivier Couture, an expert on ultrasound imaging at the National Center for Scientific Research (CNRS) in Paris. “For animal or even human studies, ultrasound imaging could now observe [products of] reporter genes in bacteria deep inside the body, a vast improvement for the field. Reporter genes are so important in light imaging that we can only dream of what they can do for ultrasound imaging.”
Shapiro and coworkers demonstrated the technique by introducing engineered bacteria into the digestive systems of mice. The researchers used an inexpensive ultrasound emitter and detector to image the bacteria in the mouse colon a few millimeters below the skin at about 10 times better spatial resolution than they could with bioluminescence. Ultrasound showed what part of the colon the bacteria landed in, whereas bioluminescence could tell the microbes were in the colon but couldn’t localize them further. The researchers also tracked tumor-homing bacteria in mice with cancer.
Caltech has filed patent applications on the technology. “We are now working on imaging bacteria in various diagnostic or therapeutic contexts,” Shapiro says, noting that his group also hopes to develop ultrasound reporter genes for mammalian cells, such as immune cells to track immunotherapy in patients.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter