Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Imaging
Watching crystal nucleation happen at atomic scale
New imaging technique adds evidence against classical models
by Sam Lemonick
June 27, 2019
| A version of this story appeared in
Volume 97, Issue 26
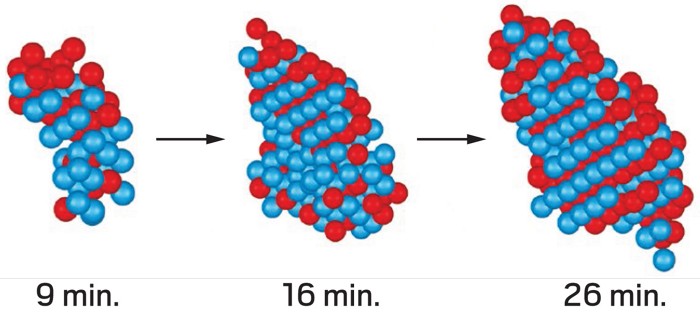
Crystals form in storm clouds, metals, drug molecules, and even in diseased tissues. Despite their ubiquity, scientists still don’t fully understand what happens when a liquid solution first starts to form a solid crystal, a step called nucleation. Now researchers have gotten their first glimpse of the details of the process, imaging individual atoms during nucleation in metal nanoparticles (Nature 2019, DOI: 10.1038/s41586-019-1317-x).
The classical model of nucleation, in which a small number of atoms form a distinct solid structure called a nucleus, the starting point for a crystal, is accurate for some systems. But in the past 2 decades scientists have found evidence for nonclassical models of nucleation. These break the classical rules in different ways—for instance, by proceeding through a two-step process starting when a disorganized but concentrated group of atoms comes together before forming a defined nucleus. No modes of nucleation have been observed with atomic-scale resolution, in part because scientists can’t be sure nuclei will form where they’re looking.
To solve that problem, Jianwei Miao of the University of California, Los Angeles, and colleagues used atomic electron tomography (AET) to observe iron-platinum alloy nanoparticles, which become crystalline when heated. In AET, a sample is rotated under an electron microscope, creating a series of 2-D images that are reconstructed to make a 3-D picture. The group followed the nucleation process through time by heating a nanoparticle at 520 °C, making an AET image, and repeating those steps twice more. By tracking each atom through the process, the researchers were able to see nucleation as it happened. What they saw was “not consistent with classical nucleation theory,” Miao says.
They found that nucleation in these nanoparticles started with a core group of a few Fe and Pt atoms adopting the ultimate, layered crystal pattern, while other atoms were progressively less ordered with distance from that core. These young nuclei could also change size, disappear, or merge with other nuclei.
The research adds to the growing evidence of nonclassical nucleation pathways, says Jeffrey Rimer of the University of Houston. Kristen A. Fichthorn of Pennsylvania State University says it isn’t surprising that the assumptions of classical nucleation theory don’t hold up exactly in these nanoparticles, but she says it’s “thrilling to observe the evolution of nuclei during phase change.” She adds that the technique reveals what until now could be seen only in simulations.
Both hope to see 4-D AET applied to other systems. Miao plans to try it in a liquid by freezing a sample at different points in the nucleation process.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter