Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Separations
Cage compounds separate deuterium from hydrogen
Material that combines small-pore and large-pore cages balances selectivity and uptake
by Bethany Halford
November 2, 2019
| A version of this story appeared in
Volume 97, Issue 43
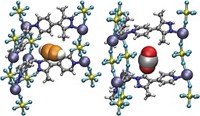
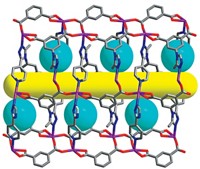
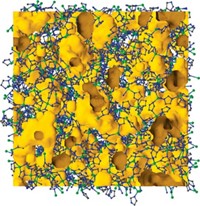
High-purity deuterium is an essential ingredient for next-generation nuclear fusion and other scientific applications, but getting D2 isn’t easy. It’s made either by electrolysis of heavy water followed by extraction or via distillation at a chilly –249 °C. Looking to sidestep these expensive and energy-intensive processes, scientists have sought to separate H2 and D2 with porous materials by a process known as kinetic quantum sieving. With this method, pores of a certain size preferentially confine the heavier isotope D2 over lighter H2 because of differences in their zero point energy—a quantum mechanical property. The problem is that the pores need to be very small, on the order of 2 Å wide, but materials with pores that small fill up quickly, so they don’t take up much D2. A team led by Andrew I. Cooper of the University of Liverpool and Michael Hirscher of the Max Planck Institute for Intelligent Systems used organic synthesis to address this problem (Science 2019, DOI: 10.1126/science.aax7427). By adjusting the apertures in structurally similar cage molecules, they were able to create cages with small pores for sieving D2 and cages with large pores for storing D2. They cocrystallized these materials, which resulted in a material that has excellent selectivity for D2 and high D2 uptake.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter