Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Structural Biology
Structures suggest how bacterial transporters bind multiple drugs
Newly revealed lipid might help export various drugs
by Celia Henry Arnaud
July 29, 2020
| A version of this story appeared in
Volume 98, Issue 30

Many bacterial cells defend themselves against antibiotics by expelling the drugs using multidrug efflux pumps. Unlike many other transporters, which only work with single compounds, such protein pumps can export a variety of drugs. The source of this so-called polyspecificity isn’t well understood.
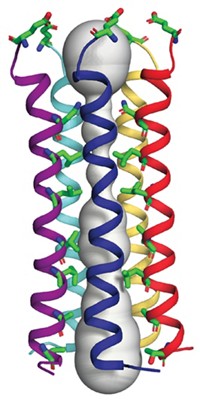
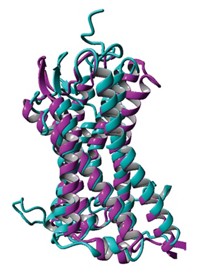
To explore the origin of the polyspecificity, Cédric Govaerts at the Université Libre de Bruxelles and coworkers solved X-ray crystal structures of a multidrug transporter called LmrP from the bacterium Lactococcus lactis with a bound ligand (Nat. Struct. Mol. Biol. 2020, DOI: 10.1038/s41594-020-0464-y). The protein is in its “outward-open” state, in which the binding pocket is open to the outside of the cell.
“LmrP is a great model to understand how transporters of the major facilitator superfamily (MFS) work,” Govaerts says. “MFS transporters are ubiquitous, as we find them in about all species.”
The new structures show that a lipid is embedded in the binding pocket along with the substrate. The lipid head group couldn’t be resolved in the X-ray structures, but a combination of molecular dynamics simulations and mass spectrometric measurements suggests that it is phosphatidylglycerol. The researchers think that the lipid may help the binding pocket adapt to different ligands, thus contributing to polyspecificity.
The researchers did not solve the structure of the protein by itself, so they don’t know whether a lipid is always there. The lipid might “just sit there all the time and adapt whenever a given substrate comes in,” Govaerts says. However, the transporter appears to bind some of the ligands in the absence of the lipid, he says.
To explore this idea, the researchers mutated the protein to disrupt lipid binding. Bacteria carrying the mutant protein pumped out drugs less effectively, and their growth was inhibited to different extents at lower concentrations of three structurally diverse antibiotics. This suggests that the lipid alters the drug pump’s activity in a substrate-specific way.
Ben F. Luisi, an expert on multidrug efflux pumps at the University of Cambridge, says these “insightful” results may help researchers gain a better basic understanding of resistance mechanisms, and may hold for other drug efflux systems. “It could also have some practical applications down the road, for instance in designing compounds that might bind avidly to the pocket and prevent drug efflux,” he says.
LmrP is the first transporter that has been found to have a functional lipid in the binding pocket, but Govaerts and his coworkers suspect that it won’t be the last. There are thousands of bacterial efflux pumps, so Govaerts is starting by using cryo-electron microscopy to solve the structures of related transporters.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter