Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Platinum Moles
Tunneling metal particles could be used to control catalyst pore size and shape
by Stephen K. Ritter
March 8, 2004
| A version of this story appeared in
Volume 82, Issue 10

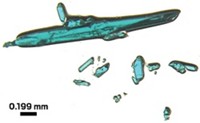
Chemists in japan who have been exploring the structural changes that catalysts undergo during operation have made a surprising observation: Platinum particles deposited on a porous zeolite support can dig out new, well-defined pores in the zeolite surface. This phenomenon could turn out to be useful as a technique to control the structure of catalysts, the researchers believe.
Hitoshi Kato of the Japan Fine Ceramics Center in Nagoya and coworkers made the discovery while observing platinum catalysts under conditions similar to those inside an automobile catalytic converter [Angew. Chem. Int. Ed., 43, 1251 (2004)]. The researchers used a [Pt(NH3)4](OH)2 solution to deposit platinum particles on zeolite ZSM-5, a SiO2-Al2O3 ceramic. The catalyst was then heated to 800 °C and exposed to an atmosphere containing trace amounts of CO, CO2, NO, H2O, and other compounds--similar to the exhaust gas of a car engine.
After 100 hours, the team examined the zeolite crystals under an electron microscope and saw that the platinum particles had vanished. The researchers subsequently found that the particles had sintered into larger particles and burrowed into the zeolite surface. The resulting pores have the same diameter as the particles, they note, and have a hexagonal cross section matching the zeolite lattice.
Kato thinks the platinum coupled with components of the exhaust gas catalyzes formation of gaseous SiO or Si(OH)4, allowing the platinum to tunnel away.
"The number of pores, as well as their shape and size, could be controlled by the diameter of the platinum particles, the duration of heating, the type of zeolite selected, and the orientation of the crystals," Kato says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter