Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Science Concentrates
July 19, 2004
| A version of this story appeared in
Volume 82, Issue 29
A small molecule in development at Bristol-Myers Squibb might be able to overcome the growing problem of resistance to the cancer drug Gleevec, according to scientists at BMS and UCLA [Science, 305, 399 (2004)]. Gleevec (imatinib) halts the progression of chronic myeloid leukemia, a type of cancer caused by overactivity of a tyrosine kinase enzyme called BCR-ABL. The drug inhibits BCR-ABL by stabilizing an inactive conformation of the enzyme. Most cases of clinical resistance to Gleevec are caused by mutations that prevent BCR-ABL from assuming this conformation. Charles L. Sawyers of UCLA and Howard Hughes Medical Institute guessed that an inhibitor that's less choosy about how it binds to BCR-ABL might be active against Gleevec-resistant mutants. He and his coworkers show that BMS-354825 (shown)--an orally bioavailable and potent inhibitor of members of a related kinase family--is effective against a majority of Gleevec-resistant mutants, both in tissue culture and in mice. BMS-354825 is now undergoing Phase I clinical trials.
Novel hyperbranched polymers containing numerous triple bonds exhibit potentially useful functional properties, according to Ben Zhong Tang and coworkers at Hong Kong University of Science & Technology [J. Phys. Chem. B., 108, 10645 (2004)]. The chemists synthesized the polyynes by polymerizing aryltriynes [Ar(C CH)3]. Polyynes with flexible hexyloxy chains on the aryl cores (shown) are soluble in solvents such as toluene and chloroform, making them readily processable by common plastic processing techniques, Tang says. The solubility of the polyynes can also be enhanced by their copolymerization with a monoyne [Ar´C
CH)3]. Polyynes with flexible hexyloxy chains on the aryl cores (shown) are soluble in solvents such as toluene and chloroform, making them readily processable by common plastic processing techniques, Tang says. The solubility of the polyynes can also be enhanced by their copolymerization with a monoyne [Ar´C CH]. Thermal curing at around 150 °C makes the materials resistant to thermal decomposition at temperatures as high as 550 °C. "The polyynes have outstandingly high light refractivity, which makes them promising candidates for photonic applications," Tang explains. The materials are also photoluminescent--that is, they emit bright blue light upon excitation. Furthermore, complexation of the polyynes with cobalt carbonyls yields cobalt-polyyne complexes that are precursors for soft ferromagnetic ceramics that may find a wide range of electromagnetic applications, Tang says.
CH]. Thermal curing at around 150 °C makes the materials resistant to thermal decomposition at temperatures as high as 550 °C. "The polyynes have outstandingly high light refractivity, which makes them promising candidates for photonic applications," Tang explains. The materials are also photoluminescent--that is, they emit bright blue light upon excitation. Furthermore, complexation of the polyynes with cobalt carbonyls yields cobalt-polyyne complexes that are precursors for soft ferromagnetic ceramics that may find a wide range of electromagnetic applications, Tang says.
Researchers have achieved detection limits about 50 times more sensitive than any previously obtained by mass spectrometry [ Anal. Chem.,76, 4484 (2004)]. The technique used by Gary Siuzdak of Scripps Research Institute and coworkers is based on a soft ionization method in which porous silicon surfaces are used to generate gas-phase ions, which are then mass-analyzed. The researchers found that modifying an oxidized porous silicon surface with fluorinated silanes improves sensitivity and also enables the surface to absorb analytes from solution selectively, via hydrophobic interactions. The technique can thus extract tiny amounts of analytes from complex matrices containing salts and other contaminants that impede MS analysis. Siuzdak and coworkers demonstrated the sensitivity of the method by using it to measure solution concentrations of des-Arg9-bradykinin, a peptide commonly used as a sensitivity standard. In a series of dilution experiments, they achieved a lower detection limit of 480 molecules, or 800 yoctomoles (800 x 10–24 moles).
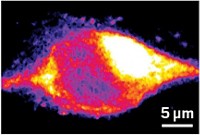
The distribution of drugs within the cellular environment plays a role in both drug efficacy and drug toxicity. Using the separation method of capillary electrophoresis with laser-induced fluorescence detection, Edgar A. Arriaga at the University of Minnesota, Minneapolis, and his coworkers have detected the cancer drug doxorubicin (shown) in individual mitochondria [J. Am. Chem. Soc., published online July 9, http://dx.doi.org/10.1021/ja0492539]. The mitochondria are monitored for doxorubicin native fluorescence and fluorescence from a mitochondrial staining agent, which is used to identify the mitochondria. By lining up the fluorescence spectra according to electrophoresis migration time, Arriaga and coworkers can identify mito chondria that contain doxorubicin. They find that not all of the mitochondria contain detectable amounts of doxorubicin. They are currently measuring doxorubicin accumulation in other organelles, including acidic organelles and the nucleus, and they plan to study the organelles from single cells.
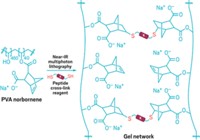
By means of the science of buckling, usually used to study crumbling buildings or the crumpling of Earth's crust, researchers at NIST and IBM have developed a quick and inexpensive way to quantitatively measure the strength and stiffness of polymer thin films [Nat. Mater., published online July 11, http://dx.doi.org/10.1038/nmat1175]. Christopher M. Stafford and coworkers first coat the polymer thin film onto a relatively thick, soft layer of polydimethylsiloxane. They then gradually stretch or compress the sample. Under this strain, the film buckles and becomes wrinkled. By measuring the space between these highly periodic wrinkles and applying straightforward buckling mechanics, the group can calculate the film's strength and stiffness. The group says the versatile technique is especially useful for determining the properties of nanoscale films, which, until now, could only be measured in relative terms rather than quantitatively.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter