Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Meeting Briefs
September 13, 2004
| A version of this story appeared in
Volume 82, Issue 37
More than 7,000 papers were presented at the American Chemical Society national meeting in Philadelphia last month. Here's a sampling of some of the research results discussed there.
- Multiborylated thiophenes as chemical sensors
- Getting at the root of lice resistance
- Self-healing fuel tank
- Oxidized guanine linked to Huntington's
- Did meteorites supply life's phosphorus?
- Buzz off
- Detection of organic phosphates
- Finding amino acid polymorphisms
- For the relief of measles
- Variants of ill-famed drug may find use in cancer war
Multiborylated thiophenes as chemical sensors
Conjugated organic polymers are finding a variety of applications as thin-film materials in electroluminescent and electrochromic devices. Polythiophenes in particular are popular because they are stable in their neutral and oxidized forms, and the polymer chain or side groups are easily modified to fine-tune the physical properties. One type of modification being studied by graduate student Anand Sundararaman, assistant chemistry professor Frieder Jäkle, and coworkers at Rutgers University, Newark, N.J., is the incorporation of Lewis acid boron centers into thiophene polymer chains or as terminal substituents of thiophene oligomers. The researchers find that if the boron atoms are substituted with electron-withdrawing pentafluorophenyl groups, blue-green luminescence is observed. In another example, ferrocenyl substituents yield a dark red polymer. Adducts can be formed between the boron Lewis acid and Lewis bases, quenching the luminescence or bringing about a color change, a property that opens up the possibility of using the materials as chemical sensors. Exposure of a borylated 3-hexylthiophene polymer to a Lewis base such as pyridine (shown), for example, quenches the material's luminescence.
As many as 12 million people worldwide are infested with lice each year. Many combat the infestation with products containing permethrin (shown). However, head lice have become resistant to this active ingredient. Understanding the basis of this resistance could help extend the effectiveness of permethrin and other lice insecticides. Toxicologists Si Hyeock Lee of Seoul National University, in South Korea, and John M. Clark of the University of Massachusetts, Amherst, have advanced the understanding of the resistance mechanism. Previously, they had found that lice resistance to permethrin arises from a set of three mutations in the  -subunit of the voltage-sensitive sodium channel of lice. Now, they have come up with a DNA-diagnostic protocol to detect these resistance-causing mutations. This tool could help establish the occurrence of resistance and allow alternative control strategies to be used to prevent resistance from advancing. Key to developing the protocol is an automated artificial membrane/blood feeding system that Clark has fashioned. It allows human head lice to be raised without human or animal hosts. With this "surrogate head," Lee and Clark have raised populations of permethrin-resistant head lice for study.
-subunit of the voltage-sensitive sodium channel of lice. Now, they have come up with a DNA-diagnostic protocol to detect these resistance-causing mutations. This tool could help establish the occurrence of resistance and allow alternative control strategies to be used to prevent resistance from advancing. Key to developing the protocol is an automated artificial membrane/blood feeding system that Clark has fashioned. It allows human head lice to be raised without human or animal hosts. With this "surrogate head," Lee and Clark have raised populations of permethrin-resistant head lice for study.
Flying the unfriendly skies could become a little safer for U.S. military pilots, thanks to scientists at Naval Air Systems Command in Patuxent River, Md. Materials engineer Christopher S. Coughlin and technician Robert F. Boswell are trying to develop a self-healing polymer that would quickly close around bullet holes. The material is highly coveted for fuel tanks in military planes and helicopters, which currently use several layers of heavy rubber for protection. The researchers work with various grades of Surlyn, DuPont's ethylene-methacrylic acid copolymer that strengthens golf balls and hockey helmets. When shot at, Surlyn seals over holes, but it degrades around jet fuel. By studying Surlyn's healing mechanism, Coughlin and Boswell hope that they can either modify the material or make other polymers that are both self-healing and fuel resistant. Coughlin says the key to Surlyn's healing powers seems to lie in the polymer's rheology, melt strength, and elasticity. A speeding bullet heats the material just enough to make the polymer snap back into place.
Like a dozen other neurodegenerative disorders, Huntington's disease results from the expansion of a string of repeated DNA trinucleotides in a disease-linked gene. The mechanism of expansion, however, remains unknown. Cynthia T. McMurray of the Mayo Clinic College of Medicine, Rochester, Minn., has data suggesting that oxidation of a guanine in the repeat region to 8-oxoguanine is the first step. Once 8-oxoguanine is formed, a cellular glycosylase enzyme is recruited to excise the damaged base, leaving a break in the DNA strand. This break would normally be sewn up by another enzyme. Instead, according to McMurray, the unusual trinucleotide repeat region tends to fold up on itself, forming a mispaired hairpinlike structure. And the repair enzyme normally recruited to deal with mispaired bases actually gets trapped on this hairpin. Entrapment of the repair enzyme allows the structure to persist long enough for a DNA polymerase to fill in the gap between the original 8-oxoguanine-induced break and the terminal end of the hairpin, thereby expanding the string of repeated trinucleotides. In mice with Huntington's disease, loss of the enzyme that excises 8-oxoguanine prevents trinucleotide expansion, she adds.
The phosphorus that's essential to biomolecules such as ATP and RNA on Earth may have come from meteorites, according to University of Arizona graduate student Matthew A. Pasek. The source of this element in biology has been a long-standing mystery. It's been known that phosphorus is abundant in meteorites, in a form known as schreibersite, which is rare on Earth. Pasek and UA assistant planetary sciences professor Dante S. Lauretta found that, by merely mixing it with room-temperature water, schreibersite could form P2O7, a biochemically useful form of phosphate. Scientists previously found that schreibersite forms P2O7, but those experiments were performed under extreme conditions, such as high temperatures.
Researchers at DuPont are developing insect repellents based on derivatives of nepetalactone, a natural product from catmint (Nepeta cataria). According to chemist Mark A. Scialdone, the insect-repellent activity of nepetalactone has been known since the 1960s. He and colleagues now find that some 3-substituted derivatives of reduced nepetalactone are better than nepetalactone itself and at least equal the potency of DEET (N,N-diethyl-3-methylbenzamide), the most widely used active ingredient in commercial products. For example, at 1% wt per vol, a 1:1 diastereomeric mixture of 3-methyldihydronepetalactone (shown) repels mosquitos better than DEET. The compounds are part of DuPont's increasing emphasis on products derived from renewable sources. Work continues at the company to develop formulations to deliver the active compounds.
Ionic liquids offer an environmentally friendlier method for detecting organophosphates, according to Chengdong Zhang and Sanjay V. Malhotra of New Jersey Institute of Technology, Newark. Many pesticides, insecticides, and chemical warfare agents are organophosphates. Their biological activity is due primarily to their inhibition of the enzyme acetylcholinesterase. A well-established assay to detect organophosphates is based on the conversion of acetylthiocholine to thiocholine and acetate by acetylcholinesterase. In the presence of dithiobisnitrobenzoate, thiocholine forms a yellow product. If the sample contains an organophosphate, no yellow product is formed. The assay is usually carried out in organic solvents, the best being cyclohexane. Using the pesticide paraoxon (shown) as the test compound, Zhang and Malhotra have shown that the assay can be done in ionic liquids, which are generally less toxic and pose a smaller environmental burden than organic solvents. In fact, the assay works even better with ethylpyridinium hexafluorophosphate than with cyclohexane.
When the word polymorphism comes up, the first thought is of single-base differences in DNA. Only 1% of these so-called SNPs, or single-nucleotide polymorphisms, result in a changed amino acid in a protein, however. Purdue University chemistry professor Fred E. Regnier has devised a method called TACT (tagging amino and carboxy terminus) to detect and identify these rare differences in proteins, called single amino acid polymorphisms (SAAPs). Identifying such polymorphisms could eventually be useful in determining which patients are likely to respond optimally to specific pharmaceutical products. In the method, control and experimental protein samples separately are digested into peptides, and each peptide is differently labeled at the carboxy and amino termini. The labeled peptides are mixed and then analyzed by mass spectrometry. In the mass spectrum, peptides found in both control and experimental samples appear as a doublet of clusters separated by an easily predicted mass difference. Unpaired clusters are the result of amino acid differences between the samples and therefore pinpoint SAAPs.
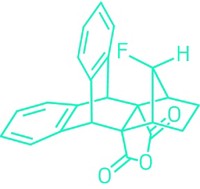
Researchers have developed nonpeptidic small molecules that inhibit entry of the measles virus into cells. Measles causes about a million deaths annually worldwide. An effective vaccine is available, but it does not always work with young infants, and other people may refuse it or may not have access to it. For the unimmunized who develop the disease, a therapeutic could bring relief, but no effective antimeasles agents are currently available. Now, postdocs Aiming Sun and Richard K. Plemper of Emory University and the collaborative team to which they belong--a group of virologists, synthetic chemists, and molecular modelers at Emory and at Ankara University, in Turkey--have identified a lead compound and a second-generation analog that prevent fusion of the virus with host cell membranes, a key step in getting inside host cells. The inhibitory potency of the second-generation agent (shown) is 260 nM. The team is currently looking for compounds with antimeasles activity in the low nanomolar range, as well as analogs that will inhibit membrane fusion by other viruses in the measles family.
Analogs of thalidomide, the drug that gained infamy for causing birth defects, could be weapons in the war against cancer. According to Milton L. Brown, an associate professor of chemistry at the University of Virginia, thalidomide analogs have strong antiangiogenic effects--that is, they inhibit the growth of new blood vessels. Antiangiogenic compounds are gaining importance as cancer chemotherapeutic agents. They halt the spread of cancer cells by starving them of a blood supply. Thalidomide itself is being used as an anticancer agent because its metabolite has such a biological activity. Brown has been designing and synthesizing analogs that have greater antiangiogenic effect than that of thalidomide. Some of the compounds he has prepared not only stop the growth of new blood vessels but also inhibit the growth of specific cancer cells themselves, such as those causing prostate cancer, colon cancer, and certain leukemias. Very few drugs with such dual action are available so far.








Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter