Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Toward Novel Lantibiotics
Enzyme may allow engineering of new antibiotics for food preservation
by Amanda Yarnell
February 2, 2004
| A version of this story appeared in
Volume 82, Issue 5

Chemists at the University of Illinois, Urbana-Champaign, have isolated and purified the bacterial enzyme responsible for turning a small peptide into lacticin 481, a member of the class of potent antibiotics known as lantibiotics. Although the genes encoding lantibiotic biosynthetic enzymes have been known for a long time, this is the first time that one of these enzymes has been purified in its active form.
"The isolation of this enzyme opens the door to possible generation of new lantibiotics with different or improved activities," notes chemist John C. Vederas of the University of Alberta.
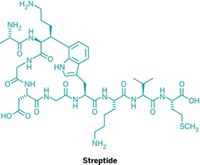
Lacticin 481 is produced by a strain of Lactococcus lactis, a bacterium used in cheese production. Other lantibiotics are used as natural preservatives in a variety of foods, including milk, meat, and canned vegetables. Unlike many antibiotics used as pharmaceuticals, lantibiotics have been used for half a century in more than 40 countries without any significant signs of lantibiotic-resistant bacteria popping up.
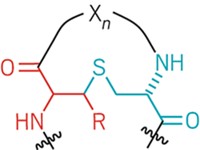
Lantibiotics are peptide-based compounds produced by bacteria that live on lactic acid. They are characterized by the presence of rings formed by two unusual double-headed amino acids--lanthionine and methyllanthionine--that contain thioether bridges.
The small enzyme LctM, which does the lion's share of synthesizing lacticin 481, was purified at Illinois by Lili Xie, Wilfred A. van der Donk, and their colleagues [Science, 303, 679 (2004)]. With the help of chemist Neil L. Kelleher, the team used mass spectrometry to prove that the purified enzyme performs a carefully controlled sequence of reactions on the linear, unmodified peptide precursor of lacticin 481.
The team found that LctM first dehydrates four specific serine and threonine residues in the peptide precursor. The enzyme then catalyzes the attack by cysteine side chains on three of these dehydrated residues, yielding a peptide containing a methyllanthionine-based ring and two lanthionine-based ones as well as a single dehydrated threonine residue. A different enzyme then trims a bit off the end of the peptide precursor to produce mature, active lacticin 481.
Remarkably, LctM can perform its string of dehydrations and cyclizations even on versions of lacticin 481 precursor peptides that are truncated or that contain different peptide subunits. "LctM's flexibility suggests that it can be used with semisynthetic substrate peptides to create new lacticin analogs," van der Donk tells C&EN.
The team also hopes to learn more about how LctM catalyzes these reactions, van der Donk says. "It will be fascinating to learn in the future how the LctM enzyme recognizes its substrate in three dimensions and the detailed mechanisms of these transformations," Vederas adds.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter