Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Science Concentrates
February 16, 2004
| A version of this story appeared in
Volume 82, Issue 7
DNA folds into octahedron
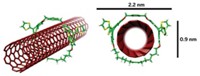
While DNA can be used to make rigid nanoscaffolding, the topologies of 3-D geometric DNA objects have prevented polymerases from copying them, making it difficult to scale up production of the molecules via cloning. Now, William M. Shih, Joel D. Quispe, and Gerald F. Joyce from Scripps Research Institute report the design and synthesis of an easily cloned, 1,669-nucleotide, single-stranded DNA molecule that folds into an octahedron in the presence of five 40-nucleotide strands of DNA [Nature, 427, 618 (2004)]. The octahedron measures 22 nm across. (Reconstruction from cryo-electron microscopy is shown; colors indicate relative electron density.) Each of the octahedron's edges is made of pairs of double helices arranged in a side-by-side manner. Shih designed the DNA object so that seven of the edges would form by paranemic cohesion, in which internal loops from the long strand wrap around one another to make a bridge without actually making or breaking any covalent bonds. Double crossovers, which resemble two double helices exchanging strands at two positions, form the other five edges via association between the long and short strands.
A new study calls into question the role of the nontraditional C 2H...O hydrogen bonds commonly observed in protein structures. Such hydrogen bonds--in which a backbone C
2H...O hydrogen bonds commonly observed in protein structures. Such hydrogen bonds--in which a backbone C 2H interacts with an oxygen atom elsewhere in the protein--are thought to be weaker than traditional hydrogen bonds. Still, the prevalence of such contacts has led some scientists to suggest that they may play an important role in stabilizing protein structures. Structural biologist James U. Bowie and his colleagues at the University of California, Los Angeles, have now provided the first experimental test of the strength of a C
2H interacts with an oxygen atom elsewhere in the protein--are thought to be weaker than traditional hydrogen bonds. Still, the prevalence of such contacts has led some scientists to suggest that they may play an important role in stabilizing protein structures. Structural biologist James U. Bowie and his colleagues at the University of California, Los Angeles, have now provided the first experimental test of the strength of a C 2H...O hydrogen bond [J. Am. Chem. Soc., published online Feb. 3,http://dx.doi.org/10.1021/ja0317574 ]. Using a C
2H...O hydrogen bond [J. Am. Chem. Soc., published online Feb. 3,http://dx.doi.org/10.1021/ja0317574 ]. Using a C 2H...O hydrogen bond in the membrane protein bacter orhodopsin as a case study, Bowie and his coworkers measured the thermodynamic stability of versions of the protein containing mutations that disrupt this nontraditional hydrogen bond. They conclude that this and many other C
2H...O hydrogen bond in the membrane protein bacter orhodopsin as a case study, Bowie and his coworkers measured the thermodynamic stability of versions of the protein containing mutations that disrupt this nontraditional hydrogen bond. They conclude that this and many other C 2H...O hydrogen bonds don't make significant contributions to the stability of proteins. Instead, Bowie suggests, many C
2H...O hydrogen bonds don't make significant contributions to the stability of proteins. Instead, Bowie suggests, many C 2H...O contacts may simply facilitate protein packing.
2H...O contacts may simply facilitate protein packing.
Although hydrogen is often touted as the fuel of the future, the gas is currently made at high cost from fossil fuels--a fact critics cite as a major stumbling block in the "hydrogen economy." University of Minnesota chemical engineering professor Lanny D. Schmidt and colleagues have built a reactor that efficiently and economically converts ethanol--a renewable fuel made from biomass--into hydrogen [Science, 303, 993 (2004)]. In Schmidt's process, an automotive fuel injector sprays wet ethanol onto the walls of the reactor tube, which are heated to about 140 °C. The wet ethanol vaporizes, mixes with air, and passes over a rhodium-ceria catalyst at around 700 °C, where it is converted into H2 and CO2. Schmidt's group reports greater than 95% conversion and nearly 100% selectivity for the catalytic partial oxidation process. Furthermore, they add that using wet ethanol would not incur the significant costs associated with removing all water from ethanol currently used as a fuel additive.
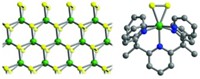
Host-guest chemistry is key to crafting a molecular necklace in which five small rings are threaded onto one larger ring [J. Am. Chem. Soc., 126, 1932 (2004)]. In the quantitative, noncovalent synthesis, five molecules of the guest compound--an electron-rich naphthalene unit (red) connected by a methylene bridge to an electron-poor dipyridyliumylethylene unit (dark blue)--assemble into the necklace's larger ring. The electron-rich end of one guest molecule and the electron-poor end of another guest molecule form a stabilized charge-transfer complex within the hydrophobic cavity of the host molecule cucurbit[8]uril (light blue), five of which comprise the necklace's smaller rings. When designing this molecular bauble, chemistry professor Kimoon Kim of Pohang University of Science & Technology, in South Korea, and coworkers predicted that the angle of the methylene bridge was close to that of an equilateral pentagon, thus determining the necklace's geometry. They speculate that by varying the length and angle of the guest molecule they could design molecular necklaces of different shapes and sizes.
Nitrogen compounds in transportation fuels can be reduced to very low concentrations using zeolite-based adsorbents under ambient conditions, according to a study conducted at the University of Michigan, Ann Arbor. In oil refineries worldwide, nitrogen and sulfur contaminants are removed from feedstocks simultaneously using hydrotreating techniques that are carried out at temperatures above 300 °C and H2 pressures of up to 100 atm in the presence of CoMo and NiMo catalysts. Hydrotreating is more effective for removing organosulfur compounds than organonitrogen species, which tend to be relatively unreactive. But now, Michigan chemical engineers Arturo J. Hernández-Maldonado and Ralph T. Yang have shown that Y-zeolite containing copper cations can remove nitrogen compounds efficiently at room temperature and pressure [Angew. Chem. Int. Ed., 43, 1004 (2004)]. Using conventional ion-exchange methods to prepare adsorbents, the team reduced the nitrogen concentration in commercial diesel fuel samples from 83 ppm by weight to well below 0.1 ppm by weight. The researchers point out that the material's adsorption capacity is readily regenerated using solvents or heat treatments.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter