Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Boron Flat out
by Stephen K. Ritter
March 1, 2004
| A version of this story appeared in
Volume 82, Issue 9

Relative to its next-door neighbor carbon, boron is one electron short. And what a difference that makes!
Carbon, with six electrons, is essential to life, while boron, with only five, is not. There are countless organic compounds having innumerable uses, but there are many fewer examples of boranes (the boron analogs of hydrocarbons) and their carborane and metallaborane derivatives. The number of applications for these boron compounds in electronics, catalysis, organic synthesis, and diagnostic and therapeutic medicine, while growing, has been limited. Nevertheless, because boron is a little different, with a diverse set of structural and bonding characteristics, chemists have remained fascinated with the prospects of striking it rich with new families of functional boron compounds, particularly all-boron clusters.
"Chemists are beginning to understand more and more about elemental boron, boranes, all-boron clusters, and boron-rich solids," notes chemistry professor Eluvathingal D. Jemmis of the University of Hyderabad, in India. "For example, solid-state chemists and physicists have shown that the properties of boron can be modified by doping its compounds with metals to form n-type or p-type semiconductors. We are beginning to learn the importance of these impurities and the specific roles they play in structural and electronic properties. If we get to a stage where we can make stabilized boron wafers or boron-rich materials to order, there would be far more applications."
Much of the understanding that Jemmis alludes to is the result of theoretical studies, where scientists imagine new compounds or isomers and then calculate the electronic structures and chemical bonding to find out whether the molecules might actually be capable of existence. Better and better computers and software in recent years have helped clear a path to the small boron clusters.
Jemmis and graduate student Elambalassery G. Jayasree recently reviewed well-recognized analogies between boron and carbon and described what they believe are some promising connections that have been overlooked [Acc. Chem. Res., 36, 816 (2003)]. The structural relationships between benzene, condensed aromatics, and graphite--as well as carbon nanotubes and fullerenes--have been a guiding principle in carbon chemistry, Jemmis says. But similar relationships between polyhedral boranes (BnHn2-, for n = 5 to 12), all-boron cluster compounds, and elemental boron allotropes are just becoming discernible, he adds.
One recent example is MgB2. This compound has been known for 50 years and is simple and inexpensive to prepare. Yet in 2001, it was discovered that MgB2's alternating graphitelike layers of boron and magnesium hexagons give rise to high-temperature superconductivity. This finding has stimulated boron cluster chemists, who are looking for other "gems" in metal borides, boron carbides and nitrides, and a host of other boron-rich compounds.
BORON HAS DEFIED conventional bonding concepts that are central to carbon compounds, Jemmis points out. "Boranes are hydrocarbon equivalents, but they come in all kinds of polyhedral structures."
Boron exhibits sp2 hybridization in most of its compounds, leaving one unhybridized p orbital unoccupied. In this bonding picture, boron has more bonding orbitals than available electrons, so it is considered "electron deficient." Boron adapts by adopting a multicentered bonding strategy that involves sharing electrons across BBB or BHB units, which necessitates formation of cluster compounds.
The most stable structures of boranes--and the related carboranes (C2Bn-2Hn)--are deltahedrons, regular polyhedrons in which all the faces are equilateral triangles. The octahedral B6H62- and icosahedral B12H122- are the most stable of these compounds, and both are analogous to benzene.
But not all boranes and carboranes are closed polyhedra: Many compounds are open frameworks in which at least one vertex of the deltahedron is missing. When enough vertices are missing, some of the molecules approach being planar, structurally similar to aromatic hydrocarbons.

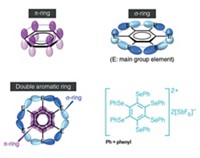
For all types of planar cluster systems, stabilization is not always achieved solely by delocalized electrons. There can also be a contribution from delocalization of electrons occupying unhybridized p orbitals in the plane of a cyclic structure. Some hydrocarbons and inorganic clusters with fully occupied p and s shells have "double aromaticity," a concept first introduced about 25 years ago by chemistry professor Paul v. R. Schleyer and coworkers. At the time, Jemmis was a graduate student in Schleyer's group at Princeton University, and later moved with Schleyer to the University of Erlangen-Nuremberg, in Germany. Schleyer now holds joint appointments at Erlangen and the University of Georgia.
When it comes to closed polyhedral boranes or other closed clusters, the three p orbitals separately form delocalized sets of related molecular orbitals, Schleyer says. This bonding picture is known as three-dimensional aromaticity, a concept that was developed by several groups, including Jemmis and Schleyer. A derivative concept called spherical aromaticity was later introduced to describe electron delocalization in highly symmetrical cage compounds, Schleyer adds.
At first, spherical aromaticity was applied to fullerenes, but now the concept is also being applied to symmetrical inorganic clusters. Recent examples predicted by Schleyer, postdoctoral researcher Zhongfang Chen, chemistry professor Andreas Hirsch of the University of Erlangen-Nuremberg, and their collaborators include the Si62- and Si122- clusters, which are antiaromatic, in contrast to the structurally similar aromatic B6H62- and B12H122- [J. Am. Chem. Soc., 126, 430 (2004); 125, 15507 (2003)].
ARMED WITH THESE newer concepts of bonding, chemists and physicists have been exploring new possibilities for small all-boron clusters. Many theoretical studies on the structures of clusters ranging from B3 to B20 have been carried out. These studies have consistently predicted that these compounds should adopt planar geometries rather than the 3-D structures of boranes and elemental boron allotropes. Yet there has been little in the way of experimental evidence to support the theoretical work.

During the past year, associate chemistry professor Alexander I. Boldyrev's group at Utah State University and physics and materials science professor Lai-Sheng Wang's group at Washington State University and Pacific Northwest National Laboratory have reported several studies on planar boron clusters ranging from B3 to B15. Some of the work is collaborative, relying on computational studies by Boldyrev's group, which includes graduate student Anastassia N. Alexandrova and undergraduate K. Alexander Birch, and gas-phase photoelectron spectroscopy studies by Wang's group, which includes postdocs Boggavarapu Kiran and Hua-Jin Zhai and PNNL scientist Jun Li.
In recent years, the Boldyrev-Wang collaboration has investigated the electronic structures and chemical bonding of a host of cluster compounds. Examples range from planar tetracoordinate carbon compounds (CAl3Si) to all-metal aromatic and antiaromatic compounds, such as NaAl4-, which has a planar aromatic Al42- unit.
"Up to this point, it wasn't clear if these planar all-boron structures were correct or why they should be planar," Wang says. "Our joint theoretical-experimental studies got us started on a serious effort to elucidate the structure and bonding of these small clusters, with the initial motivation to confirm if they are aromatic."
During the experiments, Wang's group uses a laser to vaporize a boron disk. They then isolate the various boron cluster anions that result using a time-of-flight mass spectrometer. The size-selected clusters are probed by photoelectron spectroscopy, and the results are compared with calculated parameters.
For the smallest boron clusters--B3 to B6--Boldyrev, Wang, and their collaborators have found that neutral and anionic clusters are planar and in most cases are doubly aromatic. The thermodynamically favored structures for B3 and B3- are triangles. The B4 cluster is slightly distorted from a perfect square, while B4- is somewhat more distorted and is aromatic but antiaromatic. The B42- cluster is a perfect square, similar to that observed in the all-metal aromatic compounds.
The B5 and B6 clusters reported by Boldyrev and Wang also are planar, but the additional atoms cause them to take on boatlike shapes. The theoretical and experimental work indicates that B5 and B52 are aromatic and antiaromatic, while B62 and B622 are doubly antiaromatic. "The planar B6 clusters are quite different from the octahedral B6 units often found in metal borides and boranes," Boldyrev and Wang say.
"The connection of planar boron clusters to hydrocarbons is through aromaticity and antiaromaticity," they add. "The planar boron clusters follow the 4n + 2 and 4n Hückel rules. However, the chemical bonding is more complicated because the boron clusters possess multiple and aromaticity or antiaromaticity. They should be viewed as a distinct class of chemical species."
EXPERIMENTAL EVIDENCE for double aromaticity was first established in 1997 by chemistry professor Armin Berndt of Philipps University, in Marburg, Germany, and his collaborators, which include Schleyer and Matthias Hofmann, now at the University of Heidelberg, in Germany. Berndt and coworkers have prepared planar triangular B3 or rhombohedral B4 doubly aromatic ring compounds containing hydrocarbon substituents and obtained their crystal structures.
Berndt points out that his work and that of others show that the stabilizing substituents of these "dressed" clusters don't alter their double aromaticity when compared to the "naked" all-boron versions without substituents, such as those studied by Boldyrev and Wang. These findings suggest that larger planar all-boron clusters also could be stabilized by external groups without changing their electronic structures.
When Boldyrev and Wang turned to the B7 to B9 clusters, they found a new twist in the structures: The rings are large enough that a central boron atom is needed to anchor the remaining atoms, which form a perimeter around the central boron atom. In effect, each cluster is a circular set of fused B3 triangles. The bonds from the central atom to the peripheral atoms radiate outward like spokes, leading Boldyrev and Wang to call the clusters "molecular wheels." In results about to be published, they find that the ring in the B7- cluster is "just a bit too small" for the central boron atom to squeeze in, so it sits slightly out of the plane of the ring. The B8 and B9 clusters, however, have been found to be planar [Angew. Chem. Int. Ed., 42, 6004 (2003)].
The B8 and B82- molecular wheels are perfect heptagons and B9- is a perfect octagon, they note, while the other clusters have slightly distorted structures. "Why do these clusters adopt such unusual and beautiful structures?" they ask. They find an answer in the analysis of the molecular orbitals and experimental vertical detachment energies for the valence electrons.

There are two sets of molecular orbitals for the molecular wheels, the researchers note. One set governs the peripheral BB bonding, and the other set covers the bonding between the central boron atom and the peripheral atoms. Three orbitals of the latter set are orbitals and three are orbitals, similar to those in benzene. All six orbitals are completely delocalized in the molecular plane. The B82- and B9- clusters, with six s and six p electrons, are thus doubly aromatic.
Although the localized bonds resemble the spokes of a bicycle wheel, the delocalized molecular orbitals suggest that the individual spokes can't be visualized per se, according to Boldyrev and Wang. Instead, the electron density can be envisioned as being smeared out, which might look more like a bicycle wheel spinning at a fast rate. Instead of spokes, the wheel is more like a solid disk, similar to a composite bicycle wheel.
"It may be more appropriate to call this 'disk delocalization,' relative to the cyclic delocalization in benzene," Wang and Boldyrev say. "We believe this double aromatic character is responsible for the planarity and unique coordination of these clusters."
SEVERAL YEARS AGO, Schleyer and postdoc Zhi-Xiang Wang predicted that the carborane units –C3B3–, –C2B4–, and –CB5– could replace –(CH)3– units in aromatic or antiaromatic hydrocarbons to form families of molecules they call "hyparenes" that have planar, highly coordinated carbon atoms. Two of these compounds, CB62- and CB7, which also have been studied by others, are predicted to have a carbon in the center of a ring of boron atoms, similar to boron molecular wheels. Schleyer has predicted that these planar rings could be part of larger sandwich-type compounds that resemble three-dimensional wheels.
Advertisement
Schleyer and Zhi-Xiang Wang suggested at the time that hyparene isomers containing boron or other elements in place of carbon also would be energetically favored. To date, none of the hyparenes have been synthesized, Schleyer notes. However, other types of inorganic wheel compounds are known, such as AgAu6, and planar pentagonal C2B3 carboranes are increasingly being used as ligands in multidecker transition-metal complexes.
In further expansion of the all-boron work, Lai-Sheng Wang's group recently prepared B10 to B15 planar clusters [Nat. Mater., 2, 827 (2003)]. These clusters resemble large rafts, with two to four boron atoms in the center surrounded by a peripheral ring of boron atoms.

The B10, B11-, and B12 clusters are highly stable planar compounds with six delocalized electrons, Wang says. B11- and B12, in terms of their structure and three molecular orbitals, are analogous to the cyclopentadienyl anion (C5H5-) and benzene, respectively. The B13- and B14 clusters have eight delocalized electrons and are antiaromatic, with elongated oval shapes analogous to the square-to-rectangular distortion in the antiaromatic cyclobutadiene.
"The planar B12 is in stark contrast to the ubiquitous icosahedral B12 units that are found in bulk boron and in boranes," Wang notes. The reason for the shift from the 3-D polyhedral boranes to the planar boron clusters likely stems from the bare boron atoms' lack of dangling hydrogen atoms, which stabilize the spherical aromaticity, he says. These planar boron clusters are the only series of molecules other than hydrocarbons to display this size-dependent aromatic and antiaromatic behavior, he adds.
"It appears that boron and carbon form a set of complementary chemical systems," Wang observes. "Carbon in its most stable form is characterized by the 2-D graphite system, and carbon clusters are characterized by 3-D cages, such as fullerenes. Boron's most stable form is characterized by the B12 3-D cages, and boron clusters are characterized by 2-D structures."
The confirmation that these all-boron clusters form planar wheel and raftlike shapes rather than closed, nearly spherical clusters the way boranes do "is an important landmark in chemistry," notes chemistry professor Thomas P. Fehlner of the University of Notre Dame. "It's reminiscent of the discovery when I was a graduate student that the inert gases are not inert. Discoveries such as these change our thinking in a major way.
"These results suggest that there are many more boron cluster shapes possible lying between the end points of the borane deltahedra and these planar boron clusters," Fehlner adds. "Achieving synthetic control of the ligand number and type and/or stabilizing metal fragments in metallaborane complexes will be key to generating these species in quantity."
FEHLNER'S GROUP has already shown that, in metallaboranes, choosing an early transition metal (groups 6 and 7) with fewer valence electrons instead of a late transition metal (groups 8 and 9) can lead to quite different borane structures. These complexes typically are prepared by stepwise addition of monoboranes to monocyclopentadienyl metal chlorides.
For example, Fehlner and coworkers have prepared complexes of the type (C5Me5Re)2BnHn (Me = methyl; n = 710), where the borane is coordinated to two metal atoms. The borane fragments are nearly planar in these early metal complexes, he says, rather than having deltahedral shapes observed for similar complexes made with late metals. The (C5Me5Re)2B6H6 complex, characterized by Fehlner's group as a dichloro derivative, does contain a planar B6H66- ring. This is analogous to metal-benzene coordination observed in known triple-decker complexes, he notes.
Boldyrev, Alexandrova, and Birch recently conducted an unrelated theoretical study to determine if planar boranes isoelectronic with hydrocarbons might indeed be derived from deltahedral boranes. The example they focused on was reduction of the octahedral B6H62- to planar hexagonal B6H66- [J. Am. Chem. Soc., 125, 10786 (2003)]. A similar approach to reducing homonuclear inorganic 3-D clusters to their planar analogs, such as E4 to metal-stabilized E42- (E = P, As, Sb, Bi), has existed for some time, they note.
At first glance, a highly charged species such as B6H66-, involving an element as electropositive as boron, looks unfavorable, Boldyrev notes. Yet stable, highly charged anions of more electropositive elements do exist, such as the Ga68- cluster, he says.
The Utah State team calculated several possible B6H66- structures stabilized by lithium cations. The most thermodynamically stable is Li6B6H6, a bipyramidal structure in which one Li+ cation is positioned above and one below the planar B6H66- hexagon; the remaining four cations are in the plane surrounding the hexagon. The B6H66- anion has the same calculated set of occupied molecular orbitals as benzene, Boldyrev notes.
THE RESEARCHERS also investigated the possible gas-phase, four-electron reduction of B6H62- to B6H66-, finding that this reaction should be highly exothermic. This is a surprising result, Boldyrev says, since known M2B6H6 salts have been found to be difficult to reduce, a property attributed to the stability of the 3-D aromaticity of boranes. "The exothermic reduction must stem from a balance between the aromatic bonding in the planar structure, the high repulsion of the six negative charges, and the external stabilization provided by the cations," Boldyrev says.
The researchers also calculated other species, such as Li6B5H5, Li6B7H7, and Li10B10H8, which all proved to be planar aromatic compounds isoelectronic with C5H5- (cyclopentadienyl), C7H7+, and C10H8 (naphthalene). "We believe that all deltahedral boranes can be reduced to yield stable, planar aromatic boranes," Boldyrev concludes. He expects that these compounds, once prepared, will have surprising properties, perhaps like MgB2.
The aromatic planar boron clusters also could eventually be used as ligands to form full or half-sandwich-type compounds with metal atoms, similar to cyclopentadienyl and benzene, Boldyrev and Wang note. "The most promising examples are B82-, B9-, B11-, and B12, which all have six electrons."
Wang expects that larger boron clusters beyond B15 will also be planar, but only up to a point. When the clusters become large enough, he says, the orbitals in different parts of the planar structure likely will fragment, leading to the appearance of B12 or other 3-D structures for stability.
Jemmis and his coworkers have been traveling in the opposite direction in some of their studies, looking to make connections between planar boron clusters, boranes, and larger boron structural units in bulk elemental boron. The idea is to take cues from carbon chemistry to predict potential new boron and boron-rich compounds, Jemmis says.

"I have always been fascinated with the elegant relationship between CH4 and diamond as well as between benzene and graphite," Jemmis comments. "The sp3 hybridization and the Hückel rule are cornerstones of these connections."
POLYHEDRAL BORANES are explained to a large extent by Wade's n + 1 rule for counting skeletal bonding electron pairs in boranes, where n is the number of vertices in a polyhedral borane, he explains. But the lack of a "similar connection between boranes and elemental boron has bothered me since my student days," Jemmis says. "I thought it would be ideal to link the monomeric polyhedral boranes to elemental boron, just as benzene, structurally speaking, leads to graphite."
Jemmis' group accomplished this by developing an electron-counting rule to account for condensation of boranes and related compounds into larger structures. The so-called mno rule describes the number of electron pairs needed for stable boranes, carboranes, metallaboranes, metallocenes--or any possible combination of these compounds--that have more than one polyhedral cage joined together by sharing up to four vertices. For this rule, m is the number of cages, n is the number of vertices from Wade's rule, and o is the number of single vertex-shared atoms between two cages. The mno rule shows that only certain combinations of CH and BH groups will lead to stable compounds not predicted by other counting rules.
One of Jemmis' goals has been to use the mno rule along with molecular orbital theory and X-ray structure data to sort out the electronic structure of B105 (-rhombohedral boron), the element's most stable allotrope. While carbon is found in nature as graphite and diamond, he points out, boron doesn't have any natural allotropes. Rather, the element is found in oxygen-containing minerals, such as borax. However, several allotropes of boron have been formed by thermal reduction of boron minerals or polyhedral boranes.
Since the icosahedral B12H122- is the most stable borane anion, Jemmis explains, it's natural for nature to construct elemental boron from the B12 icosahedron, which turns out to be the simplest boron allotrope (-rhombohedral boron). All other boron allotropes are built of B12 units connected to one another through additional interstitial boron atoms.
The B105 structure is made up of a B84 supercluster. Three B84 units are conjoined by B10 units and a single bridging boron atom to give the 105-atom unit cell. -Rhombohedral boron has interesting properties, Jemmis notes. It melts at about 2,450 °C, is stronger than steel, is harder than corundum, but is lighter than aluminum. It acts as a p-type semiconductor and can be made an n-type semiconductor by doping with metal atoms. Thus, there are many potential applications for B105 and other boron allotropes as structural and electronic materials.
Advertisement
Jemmis and coworkers have shown that breaking the -rhombohedral structure into different-sized units generates new structural types, such as B57 (B28BB28) and B48. On a different level, the B84 unit can conceptually be thought of as a B12 unit trapped inside a B72 unit, much like atoms encapsulated inside the cavity of a fullerene. Removing the central B12 leads to a structural and electronic connection between B72 and the C6012- fulleride, a species that has been isolated as C60Li12 and C60K12. The B72 unit can hypothetically be converted into C6012- by replacing boron atoms with carbon atoms. These types of exercises are useful in predicting potential new boron and boron-rich compounds, Jemmis says.
Schleyer and Zhi-Xiang Wang developed a variation of the borane electron-counting rules--the 6m + 2n rule--to predict a new class of similarly large boranes and carboranes in which the polyhedral cage structures with protruding hydrogen atoms resemble spiny sea urchins [J. Am. Chem. Soc., 125, 10484 (2003)]. The number of skeletal electrons needed for the hypothetical compounds, built up by replacing carbon atoms with boron atoms, are derived from the number of m faces larger than triangles and n triangles. The rule predicts stable compounds such as B92H928-, which is derived by starting with C60.
Experimental work to build macropolyhedral boranes by fusing together small stable boranes provides other support for the possible controlled synthesis of larger all-boron compounds. Chemistry professor John D. Kennedy and coworkers of the University of Leeds, in England, have synthesized globular metallaboranes they call "megaloboranes"--compounds with small all-boron cluster cores surrounded by a metal-containing borane skin. These compounds have led the researchers to speculate that larger megaloboranes would have larger all-boron cores [Pure Appl. Chem., 75, 1239 (2003)].
Kennedy's group has calculated that B27H21, with a seven-atom boron core, and B84H54, with a 24-atom boron core, should be stable. They propose that compounds of this nature could be synthesized by assembling borane units around a central borane core. Another possibility they suggest is to prepare them by laser ablation of elemental boron in a hydrogen atmosphere. Although the focus of the Leeds researchers is on preparing large boranes, subsequent elimination of the hydrogen atoms as H2 or a method for the megaloboranes to shed their skin to leave the cores might be possible. In this case, the boron cores would need to be stabilized to counter excessive negative charges, for example, by incorporation of metal centers, Kennedy says.
"It should be possible," Jemmis says, "to design polyhedral boranes and even elemental boron allotropes based on other fullerenes with the right electron count, even though individual compound stabilities will depend on many factors. Thus, like other carbon compounds, even the fullerenes are not so far away from boron after all. Nature has yet to unveil many secrets in this area."





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter