Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Science Concentrates
March 21, 2005
| A version of this story appeared in
Volume 83, Issue 12
Additional support for magnesium ion having a flexible inner coordination sphere comes from a new study (Biochemistry 2005, 44, 4877). Computations by Stefan Kluge and Jennie Weston at Friedrich Schiller University, Jena, Germany, indicate that generating a hydroxide ion by deprotonating one of six water molecules around Mg2+ triggers a spontaneous change from hexa- to pentacoordination (shown). Formation of metal-bound hydroxide from metal-bound water is key to the action of metallohydrolases, Weston says. Conformationally flexible Zn2+ is often the metal in those enzymes; that Mg2+ can replace Zn2+ hints that Mg2+ could be flexible as well. A report by Northwestern University researcher Douglas M. Freymann of a GTPase in which Mg2+ is pentacoordinated suggests that "pentacoordinated Mg2+ is a biochemical reality," Weston says. A connection between the new results and the GTPase work is "speculative," Freymann comments. Nevertheless, the new work could encourage new ways of thinking about magnesium chemistry in biological systems, he adds.
The first global measurements of methane in the lower troposphere reveal a surprising abundance of methane over tropical rainforests (Science, published online March 17, http://dx.doi.org/10.1126/science.1106644). After CO2, methane is the second most important greenhouse gas affected by human activity. Researchers led by Christian Frankenberg at the University of Heidelberg, in Germany, determined global levels of methane from data collected between August and November 2003 by a spectrometer aboard the European Space Agency's environmental research satellite, which measures the intensity of solar radiation reflected from Earth. They conclude that current atmospheric models have underestimated methane from tropical rainforests by as much as 4%. Wetlands, biomass burning, cattle, termites, or a hitherto unknown source could be the missing methane contributor, Frankenberg says. Better estimates of current methane distribution will greatly improve climate models, he adds.
The functions of conserved amino acids at the interface between two halves of a sandwich protein have been determined. Various protein families adopt the sandwich configuration, in which two main ß-sheets pack face to face. In nearly all sandwich proteins, eight amino acids are conserved at the interface; why that is so has been unknown. Researchers have suggested that these could play either mechanistic or energetic roles, by guiding protein folding or by stabilizing the final, folded structure, respectively. Now, Corey J. Wilson and Pernilla Wittung-Stafshede at Rice University, after studying the eight amino acids in a model sandwich protein, bacterial apoazurin, find that the conserved residues adopt both roles (Proc. Natl. Acad. Sci. USA 2005, 102, 3984). "Half the residues form nativelike interactions in the folding transition state, whereas the other residues instead govern high native-state stability but are not part of the folding transition state," Wittung-Stafshede says. She believes the findings will apply to other sandwich proteins as well.
A nanoporous crystalline molecular material held together by weak van der Waals interactions has a high storage capacity for methane and carbon dioxide under mild conditions of ambient pressure and temperature (Angew. Chem. Int. Ed. 2005, 44, 1816). Piero Sozzani and coworkers at the University of Milan-Bicocca, in Italy, measured the absorption capacity of crystals of tris-o-phenylenedioxycyclotriphosphazene for gases such as argon, nitrogen, oxygen, hydrogen, CH4, and CO2. The crystal (shown), which has hexagonal channels and permanent nanoporosity, can store large amounts of CH4 and CO2 selectively over N2, O2, and H2. This property suggests applications in fuel storage, H2 purification, and CO2 removal from air, the authors say. Using special techniques, they showed that the gas molecules are in close contact with the walls of the nanopores. The multiple aromatic receptors lining the channel walls and the tight fit with the guests recall the specificity of biological nanochannels, the authors observe.
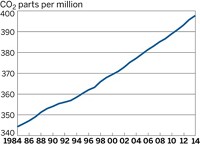
Scientists generally accept that global temperatures will continue to rise as long as the atmospheric concentration of greenhouse gases goes up. In Science, however, two articles argue that the heat capacity of the oceans, which is greater than the atmosphere's, will cause global temperatures to keep rising even if the atmospheric composition of greenhouse gases stay fixed at today's level. With a relatively simple climate model, Tom Wigley of the National Center for Atmospheric Research (NCAR) finds that the global average temperature would rise 0.2 to 1 °C by 2400 with the atmospheric composition fixed at the current level (Science 2005, 307, 1767). On the other hand, if greenhouse gas emissions were kept constant at today's high level, global temperatures would rise 2 to 6 °C by 2400, and average sea level would rise 7 to 50 cm per century, he reports. Gerald A. Meehl and colleagues at NCAR, using more complex climate models, report that by 2100, global temperatures would rise about 0.5 °C and average sea level would go up about 10 cm if atmospheric greenhouse gas concentrations were stabilized at the current level (Science 2005, 307, 1769).
Advertisement




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter