Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
VX Disposal Begins
Army holds off on shipping hydrolysate from Indiana to New Jersey for secondary treatment
by LOIS R. EMBER, C&EN WASHINGTON
May 23, 2005
| A version of this story appeared in
Volume 83, Issue 21

Earlier this month, Army contractors began destroying the deadly nerve agent VX stored in bulk in nearly 1,700 hardened, one-ton steel containers at the Army's Newport (Ind.) Chemical Depot. The stockpile of 1,269 tons--or about 250,000 gal--of VX constitutes 4% of the U.S.'s originally declared chemical weapons arsenal. It will take more than two years to destroy the arsenal at an estimated cost, from design to closure of the facility, of $1.3 billion.
The liquid agent is being destroyed by caustic neutralization. On the first day of destruction, May 5, workers from Parsons drained two containers and transferred the combined 360 gal of VX to a holding tank. The next day, about 90 gal of VX (8% by weight) was pumped into each of two 1,000-gal reactors, mixed with water and sodium hydroxide, and heated to 194 F.
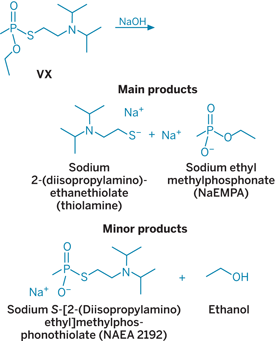
Samples taken periodically from the reactors were analyzed using gas chromatography/mass spectrometry at an on-site lab. Test results confirmed that constant agitation of the hot caustic mixture over a six-hour period had successfully hydrolyzed VX's PS bond. Residual VX--O-ethyl S-[2-(diisopropylamino)ethyl]methylphosphonothioate--in the resulting mixture was detected at 14 ppb; the Army considers #20 ppb of VX a safe level.
The VX neutralization on May 6 produced about 2,000 gal of a caustic wastewater--hydrolysate--which will be stored temporarily on-site in 5,000-gal intermodal steel containers. "These containers consist of a cylindrical tank mounted within a protective rectangular steel framework," explains Army Chemical Materials Agency spokesman Jeffrey Lindblad, and they can be used to transport the hydrolysate off-site for further treatment.
Under an Indiana permit, Newport can store up to 340,000 gal of hydrolysate. Lindblad says it will take Newport "at least a year to reach its permitted storage capacity." Storage is necessary for several reasons.
Caustic hydrolysis does not cleave the CP bond of VX, and the resulting hydrolysate contains a mixture of two major components--sodium ethyl methylphosphonate (NaEMPA) and sodium 2-(diisopropylamino)ethanethiolate (thiolamine)--from the main reaction. A side reaction splits the remaining VX into sodium S-[2-(diisopropylamino)ethyl]methylphosphonothiolate or NaEA 2192, which has some nerve agent activity, and ethanol. NaEA 2192 is further hydrolyzed to thiolamine and disodium methylphosphonic acid (Na2MPA).
Because VX is not completely hydrolyzed to harmless components, it is not considered destroyed under the terms of the chemical weapons treaty, so secondary treatment is necessary. Initially, the Army was going to use supercritical water oxidation on-site to destroy the organic components.
The Army encountered problems with the planned oxidation and decided, especially after the terrorist attacks of Sept. 11, 2001, to send the hydrolysate off-site to a commercial wasterwater treatment facility for further treatment and ultimate disposal. Its plans to send the hydrolysate to a Dayton, Ohio, facility were derailed by public opposition. The Army then turned to DuPont's Secure Environment Treatment (SET) facility in Deepwater, N.J., to treat the estimated 4 million to 6 million gal of hydrolysate that will be produced.
THE ARMY envisioned DuPont trucking about 5,000 gal per day of hydrolysate from Newport to Deepwater. Once at the SET facility, the hydrolysate would be pretreated with hydrogen peroxide to oxidize the thiolamine and get rid of the obnoxious odor. The pretreated hydrolysate would then be put through DuPont's patented treatment process involving biological oxidation and powdered activated carbon. The solids in the effluent would be allowed to settle, then dewatered and buried in DuPont's permitted on-site hazardous waste landfill. The remaining effluent would be combined with other treated plant wastes and discharged to the Delaware River.
Last year, DuPont conducted a treatability study and concluded, chemical engineer Todd R. Owens says, that discharging the treated waste "had no impact on the river." Delaware's Department of Natural Resources & Environmental Control (NREC) thought otherwise.
According to NREC environmental engineer Richard W. Greene, the state had several concerns about the effluent that DuPont planned to discharge to the river. It would contain a "complex mixture of wastes, including heavy metals," which DuPont didn't adequately address in its treatability study, Greene says. Moreover, the peroxide pretreatment would eliminate the thiolamine but would have no substantial effect on phosphonate (EMPA and MPA) levels.
The very water-soluble phosphonates are not much affected--that is, oxidized--by the subsequent biological treatment or absorbed onto the activated carbon. Although the discharged concentrations of the phosphonates would not be at toxic levels, the state was concerned about their toxicity in combination with the other compounds discharged, including trace amounts of VX, stabilizers added to VX during storage, and heavy metals. Delaware was also concerned about the "potential that phosphorus from the phosphonates might be released" and stimulate oxygen-depleting algae blooms, Greene says. "The algae effects were brushed off by DuPont," he adds.
Greene readily admits that DuPont "took our concerns to heart" and has developed a phosphonate removal treatment. DuPont's Owens says the removal method involves substituting the much stronger oxidizer sodium persulfate (Na2S2O8) for hydrogen peroxide in the pretreatment step. "EMPA is converted to MPA, which is precipitated out using ferric chloride. The resulting solids are then filtered and put into our on-site Resource Conservation & Recovery Act-permitted landfill," Owens explains. These steps, he says, "eliminate 95-99% of the phosphonates."
The remaining solution then passes through the biotreatment/powdered-activated-carbon facility to remove remaining organics, and the final effluent is mixed with other treated commercial wastewater and discharged into the river.
Data on the phosphonate removal technology were not available for the Centers for Disease Control & Prevention's review, which it delivered to the Army in early April. CDC is now reviewing the removal process, which could take months.
Delaware and New Jersey governors have opposed shipping the hydrolysate to the Deepwater facility, declaring that the wastewater should be treated on-site or, at least, closer to Newport. Last year, senators and representatives from both eastern states asked CDC to assess the safety of transporting the hydrolysate and the human health risks associated with handling, shipping, and treating the wastewater. CDC turned to the Environmental Protection Agency for an assessment of the ecological hazards of the Army's plan.
In its April report to the Army, CDC concluded that destruction of DIC (diisopropylcarbodiimide)-stabilized VX can be safely done at 8% agent loading, which the Army demonstrated on May 6. According to Lindblad, the Army now believes that about 60% of the VX stockpile was stabilized with DIC to prevent the nerve agent from deteriorating during storage. The remaining 40% was stabilized with DCC (dicyclohexylcarbodiimide) or a combination of DIC and DCC.
CDC initially had no data to evaluate safe destruction of DCC- or DIC-DCC-stabilized VX at either 8% agent loading or the higher 16% agent loading the Army eventually hopes to achieve. Lindblad says the Army conducted "additional testing on all stabilized agent last fall and winter. The results of the tests indicate that we can safely destroy the entire stockpile at 8% and 16% agent loading." Safely means that residual VX levels will be below 20 ppb. Lindblad says, "The Army has provided those results to CDC," which is now evaluating the results along with DuPont's phosphonate removal process.
Also in the April report, CDC said that the hydrolysate could be safely handled and transported, provided proper precautions are in place both at Newport and in transit to an off-site wastewater treatment plant. Furthermore, CDC, with some reservations, said DuPont's SET process is viable and should be capable of treating the major components of the hydrolysate.
EPA, however, concluded that DuPont's ecological risk assessment of the peroxide pretreatment/biological treatment was inadequate. It was not possible, EPA said, to determine whether discharge of the treated effluent to the Delaware River is an acceptable risk. Further, EPA noted that the Army's acceptable safety level of 20 ppb for VX in the hydrolysate is based "solely on the protection of humans from a drinking water source and may not be protective of aquatic organisms through ingestion or dermal exposure."
CDC found that the Army could safely move ahead with destroying DIC-stabilized VX at 8% agent loading. But "CDC cannot recommend proceeding with the treatment and disposal at the DuPont SET facility until EPA's deficiencies are addressed," the center's report said. This put a halt, at least temporarily, to the Army's plan to ship the hydrolysate off-site for further treatment and disposal.
Meanwhile, Perma-Fix Environmental Services announced a mobile, on-site process that it claims can treat VX at room temperature without producing caustic hydrolysate. The company has released little information but says its process uses chemical oxidation and converts phosphorus-bearing compounds to mineralized metal salts. Subsequent biological treatment converts organic compounds to carbon dioxide and water, which is recycled back into the process.
Lindblad says Perma-Fix has "indicated a desire to compete for work at Newport if the Army reopens competition," which is not likely at this time. The company has not "submitted its technology for review by the Army," and switching horses in midstream would add millions of dollars and years to the project, he says.
FURTHERMORE, Lindblad says that "the Army and DuPont are working with CDC and EPA to address the ecological concerns," and the Army and DuPont believe that the company's "phosphonate removal technology will address the concerns." Public officials think otherwise.
Delaware's congressional delegation, both Democrats and Republicans, support continued study "before the Army can even consider moving forward with this project." As Greene of Delaware's NREC explains: "Although the new technology looks promising, Delaware is not currently in a position to give the project an unqualified green light. We await CDC's and EPA's supplemental review."
Rep. Robert E. Andrews (D-N.J.) says he "remains adamantly opposed" to transporting the hydrolysate to New Jersey. Andrews, Rep. Jim Saxton (R-N.J.), and Rep. Frank A. LoBiondo (R-N.J.) have inserted language in the Pentagon's fiscal 2006 authorization bill that prevents any shipment until the Army certifies to Congress that CDC and EPA have found no substantial risk to the Delaware River or to human health.
DuPont Chemical Solutions Enterprise Vice President & General Manager Nick C. Fanandakis says DuPont "would only be involved in the Army's project if it can be accomplished safely and effectively without any adverse impact on the community, our employees, or the environment."






Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter