Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Carbene Ligands Diversified
Cyclic alkyl-amino carbenes expand scope of Pd-catalyzed coupling reactions
by Stephen K. Ritter
August 8, 2005
| A version of this story appeared in
Volume 83, Issue 32
CATALYSIS
A new family of cyclic carbene ligands has been synthesized, expanding the arsenal of these popular ligands and opening a door to homogeneous catalysis of a broader range of chemical reactions.
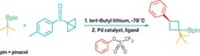
The cyclic alkyl-amino carbenes (CAACs) were prepared by Vincent Lavallo, Guy Bertrand, and their coworkers at the University of California, Riverside (Angew. Chem. Int. Ed., published online Aug. 1, dx.doi.org/10.1002/anie.200501841). CAACs are five-membered rings with a nitrogen atom adjacent to the carbene carbon on one side and a quaternary carbon atom adjacent to the carbene on the opposite side.
"The design of this new class of ligand is quite interesting since, for the past 10 years, the field of carbene ligands has been dominated by cyclic diamino ligands, also known as N-heterocyclic carbenes [NHCs]," observes Cathleen M. Crudden of Queen's University, Kingston, Ontario.
"The advantages of the new ligands are their very strong electron-donating power, their facile synthesis, and the fact that ring substituents can be placed in very close proximity to the metal," Crudden adds. "These steric properties have an important influence on the activity of the resulting metal complexes."
Bertrand's group considered that replacing one of the electronegative nitrogen atoms of NHCs with an alkyl carbon might improve the electron-donating power of the ligands toward transition metals. It turns out that the donor properties are stronger than those of standard phosphine ligands and most NHCs, Bertrand notes.
The steric environment makes CAACs different from classical types of ligands, he says. When the quaternary carbon is part of a cyclohexane ring with pendant methyl and isopropyl groups, the cyclohexane is locked into a conformation that provides a "wall of protection" for the metal complex to catalyze reactions with relatively small reactants. When an unsubstituted cyclohexane is used, the cyclohexane ring can flip conformations in solution to accommodate bulkier reactants.
The researchers show that CAAC palladium complexes are efficient for  -arylation of ketones with unactivated aryl chlorides at room temperature--reactions that haven't been possible before under these mild conditions. They also accomplished
-arylation of ketones with unactivated aryl chlorides at room temperature--reactions that haven't been possible before under these mild conditions. They also accomplished  -arylation of aldehydes with aryl chlorides for the first time--reactions that had been hampered by aldol condensation side reactions.
-arylation of aldehydes with aryl chlorides for the first time--reactions that had been hampered by aldol condensation side reactions.
"Working out the electronic and steric variations in phosphine chemistry kept inorganic chemists happy for decades," comments Robert H. Crabtree of Yale University. "The NHC framework allows much more variability, and new types of NHCs are emerging on a regular basis. Bertrand has come up with something very promising here."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter