Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Science Concentrates
November 21, 2005
| A version of this story appeared in
Volume 83, Issue 47
Aspirin Polymorph Found
In the late 1960s, there were indications that aspirin might have a second crystalline form, but it continued to escape detection. Now, Michael J. Zaworotko, chemistry professor at the University of South Florida, Tampa, and coworkers at South Florida and TransForm Pharmaceuticals, Lexington, Mass., have found this elusive polymorph (J. Am. Chem. Soc. 2005, 127, 16802).
This second form was obtained during cocrystallization experiments with aspirin and other compounds. Form II is kinetically stable at 100 K, but it converts back to form I at ambient conditions. Both forms contain a hydrogen-bonded carboxylic acid dimer (shown). But they differ in the way the dimers adjoin one another through their acetyl groups: The familiar form I assembles into dimers of dimers, whereas the new polymorph forms chains of dimers.
Biomolecules at unprecedented resolution
A microscopy system with sufficient resolution to observe atomic-level mechanisms of biomolecular phenomena has been designed and developed by Steven M. Block of Stanford University and coworkers (Phys. Rev. Lett. 2005, 95, 208102). The device is an ultrastable optical trap that uses infrared radiation to observe and control biomolecular forces. Block and coworkers demonstrated its capabilities by using it to observe folding and unfolding of DNA hairpins and to study the mechanism by which RNA polymerase transcribes DNA (Nature, published online Nov. 13, dx.doi.org/10.1038/nature04268). Some of the studies’ measurements were accurate to 1 Å, the highest resolution observations ever made on an individual protein, according to Block. The RNA polymerase study sheds new light on the issue of whether the enzyme moves along DNA in a continuous, one-residue-at-a-time manner or in a discontinuous way during transcription. The idea “that RNA polymerase climbs the DNA ladder one base pair at a time is probably the right answer,” Block says.
Organic reaction network claims predictive power
Computational analysis of organic syntheses reported between 1850 and 2004 reveals that a set of stochastic equations describes the interconnectivity of the many reactions and molecules and in turn allows for predictions to be made about future developments in organic chemistry (Angew. Chem. Int. Ed. 2005, 44, 7263). Bartosz A. Grzybowski and his colleagues at Northwestern University studied millions of organic syntheses that have been deposited in the MDL CrossFire Beilstein database. In graphical form, the data appear as an abstract network with a node structure similar to that of the World Wide Web. Highly connected nodes represent synthetically more useful compounds, analogous to cities that function as airline hubs. The team reduced the data to a set of equations that uses the interconnectivity and molecular masses to track the evolution of organic chemistry and make useful predictions, Grzybowski says. Among the possible predictions are how many compounds will be made in the future, the most probable masses of products for planned reactions, how pharmaceutically or industrially important the compounds might become, and how much they might cost.
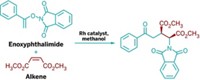
Asymmetric Biginelli reaction
A new chiral catalyst based on ytterbium enables the room-temperature formation of optically active dihydropyrimidines through multicomponent Biginelli condensation with good to excellent enantioselectivities.
In the Biginelli reaction, an aldehyde, a 1,3-ketoester, and a urea or thiourea condense in one pot to form polyfunctionalized dihydropyrimidines. Many of these heterocyclic compounds possess potent biological activities, and researchers have long sought an asymmetric version of the atom-economical reaction. Yijun Huang, Fengyue Yang, and Chengjian Zhu of Nanjing University, in China, now have developed an asymmetric version catalyzed by ytterbium triflate [Yb(OTf)3] with a chiral hexadentate ligand (J. Am. Chem. Soc. 2005, 127, 16386).
They applied the optimized protocol to monastrol, the only small molecule known to inhibit mitosis by interacting with the kinesin motor protein Eg5, and to (R)-SQ 32,926, a potent orally active antihypertensive agent. The reaction of m-hydroxybenzaldehyde, acetoacetate, and thiourea yielded (R)-monastrol in 99% enantiomeric excess. (R)-SQ 32,926 was obtained in more than 99% enantiomeric excess. The catalyst can be reused several times without significant loss of activity after recovery by extraction, the authors report.
“Accomplishing a direct enantiocontrolled route to dihydropyrimidines has been a challenge for synthetic chemists for many years, and this work will benefit ongoing efforts in drug discovery in industry and academe,” comments chemistry professor Peter Wipf of the University of Pittsburgh. Wipf also points out that the new study complements a recent one by Scott E. Schaus at Boston University. Schaus and coworkers obtained similar polyfunctionalized heterocycles in good enantiomeric excess by cyclizing chiral Mannich addition products (J. Am. Chem. Soc. 2005, 127, 11256).
Whither missing xenon?
The levels of xenon in the atmospheres of Earth and Mars are much lower than those of other noble gases, a puzzle known as the missing xenon problem. Potential hiding places for xenon include ices, clathrates, or sediments, but scientists haven’t found it yet. Now, geophysicist Chrystèle Sanloup at the University of Pierre & Marie Curie, in Paris, and colleagues show that, at high temperatures and pressures, the normally unreactive element can bond covalently with oxygen in quartz (Science 2005, 310, 1174). This discovery suggests that xenon could exist in stores of quartz that lie deep inside Earth, a possibility that would normally escape notice because xenon diffuses out almost immediately at surface conditions. The group placed quartz and xenon in a platinum cell, pressurized and heated it, and studied the contents with synchrotron X-rays and scanning microscopy. The xenon, they believe, displaces silicon atoms in the crystal lattice.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter